Fig. 3.
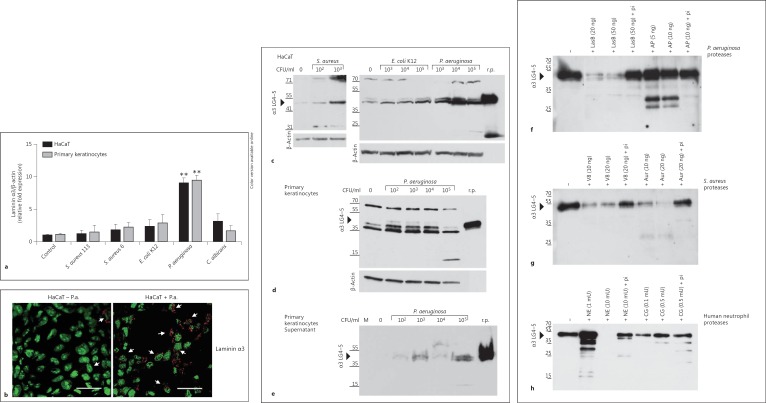
Laminin α3 chain and α3 LG4-5 expression in keratinocytes is upregulated following pathogenic challenge and bacterial/neutrophil proteases are able to cleave α3 LG4-5. a HaCaT keratinocytes and primary human keratinocyteswere analyzed by real-time-PCR 6 h after infection with various bacterial pathogens [S. aureus 113, S. aureus 6 (clinical isolate 6), E. coli K12 and P. aeruginosa] and the yeast C. albicans. The fold induction of human laminin α3 expression was normalized to β-actin, compared to the uninfected cells (control) set as 1. Means ± SD of 3 independent experiments are shown. ** p < 0.01. b HaCaT cells were analyzed by confocal microscopy for laminin α3 expression 6 h after infection with 1 × 106 CFU/ml P. aeruginosa (HaCaT + P.a.) compared to the uninfected HaCaT cells (HaCaT - P.a.). The cell nuclei are green and the laminin α3 is red. Arrows indicate slight expression of laminin α3 in uninfected cells and increased expression in infected cells. Bar: 30 μm. Colors refer to the online version only. c-h One gel, representative of >3 experiments performed. c-e Recombinant α3 LG4-5 (r.p.; 20 ng) served as a control. c Western blot of confluent HaCaT cells infected with S. aureus 113 (0, 102 and 103 CFU/ml), E. coli K12 (0, 103, 104 and 105 CFU/ml) or P. aeruginosa (103, 104 and 105 CFU/ml) for 6 h indicating expression of laminin α3 LG4-5 (approx. 40 kDa) in cell lysates. β-Actin served as a loading control. d Laminin α3 LG4-5 expression in cell lysates of primary keratinocytes in response to P. aeruginosa (102, 103, 104 and 105 CFU/ml) infection for 6 h compared to uninfected cells (0 CFU/ml). β-Actin served as a loading control. e Secretion of α3 LG4-5 into supernatant shown by immunoblot of cell culture supernatants from primary keratinocytes infected with increasing concentrations of P. aeruginosa compared to uninfected cells (0). Control media alone (M) was applied. f, g Recombinant α3 LG4-5 (approx. 15 ng) was incubated with purified proteases from P.aeruginosa, i.e. elastase B (LasB) or alkaline protease (AP) (f), or the metalloprotease aureolysin (Aur) and the serine proteinase V8 from S. aureus (g) for 60 min at 37°C. h Recombinant α3 LG4-5 (approx. 15 ng) was incubated with increasing concentrations of NE (1, 10 mU) or cathepsin G (CG; 0.1, 0.5 mU) in PBS for 60 min at 37°C. Addition of protease inhibitor cocktail (pi) abolished proteolytic activity. Proteolytic processing was analyzed by Western blot using a polyclonal antibody to probe α3 LG4-5.