Abstract
IP3-mediated Ca2+ release is a crucial neuronal signaling mechanism that has not been extensively characterized in the mammalian cerebral cortex. We used two-photon, video-rate microscopy to image Ca2+ signals evoked by photoreleased IP3 in pyramidal neurons of mouse prefrontal cortex. Ca2+ responses to photoreleased IP3 varied greatly between different neurons; however, within IP3-responsive neurons, the soma invariably showed highest sensitivity, with signals increasing nonlinearly with [IP3]. Responses to paired photorelease displayed inhibition, whereas IP3-evoked Ca2+liberation was potentiated by Ca2+ entry during action potentials and vice versa. IP3-mediated Ca2+ signals strongly inhibited spike firing through activation of K+ membrane conductance. Metabotropic signaling via the phosphoinositide pathway thus serves as a powerful and sustained modulator of excitability in cortical neurons and displays complex reciprocal interactions between electrical and chemical signals.
Keywords: calcium signaling, coincidence detection, electrophysiology, flash photolysis, modulation, prefrontal cortex
Introduction
Intracellular Ca2+ ions play vital roles in regulating numerous aspects of neuronal signaling (Berridge, 1998). This Ca2+ arises from two sources: influx of extracellular Ca2+ through voltage- and ligand-gated plasma membrane channels, and liberation from intracellular stores, such as the endoplasmic reticulum (ER). Although much is known about the influx pathways (Berridge et al., 2000;Sabatini et al., 2001), the importance of Ca2+ liberation from intracellular stores has only recently become apparent (Meldolesi, 2001; Rose and Konnerth, 2001). Ca2+ liberation from the ER is mediated through ryanodine receptors (RyR) and inositol trisphosphate (IP3) receptors (IP3Rs) (Berridge, 1998). Both function as Ca2+-permeable channels in the ER membrane, the opening of which is promoted by cytosolic Ca2+, leading to a phenomenon of Ca2+-induced Ca2+ release (CICR) (Friel and Tsien, 1992; Nakamura et al., 1999). Moreover, in addition to Ca2+, the gating of IP3Rs requires IP3, an intracellular messenger that is generated through a Gq-coupled signal transduction pathway. Ca2+ and IP3 can thus function as “chemical” computational signals, mediating complex, bidirectional interactions between the plasma membrane and ER that underlie diverse processes, including excitability, neurotransmitter release, plasticity, and gene transcription (Berridge, 1998).
Despite the fact that the phosphoinositide system is particularly well developed in brain (Berridge, 1998), most of our knowledge of its functioning derives from studies in nonexcitable cells. Key findings include the inhibitory and excitatory influences of cytosolic Ca2+ on Ca2+liberation through IP3Rs (Iino, 1990;Bezprozvanny et al., 1991; Finch et al., 1991; Yao and Parker, 1992;Ilyin and Parker, 1994) and the hierarchical spatial arrangement of Ca2+ liberation as “elementary” events that can serve both as local signals and become coordinated to propagate global Ca2+ waves (Yao et al., 1995; Berridge, 1997).
The dual requirement for IP3 and cytosolic Ca2+ to promote additional Ca2+ liberation (Bezprozvanny et al., 1991; Finch et al., 1991) suggests that the IP3receptor may serve as a coincidence detector (Berridge, 1998; Rose and Konnerth, 2001) and therefore play a role in synaptic plasticity (Fujii et al., 2000; Miyata et al., 2000; Nishiyama et al., 2000). Ca2+ released from intracellular stores can also have short-term effects on membrane excitability (Morikawa et al., 2000; Yamamoto et al., 2002), as well as potential long-term implications by regulating gene transcription (Mellstrom and Naranjo, 2001).
Although it is clear that IP3-sensitive Ca2+ stores are involved in many aspects of neuronal functioning, little is known regarding its role in the frontal and prefrontal cortex, despite that fact that this region contains a significant level of IP3 receptors (Sharp et al., 1999), Gq-coupled receptors (Wilson and Minneman, 1989; Levey, 1993), and Ca2+-activated conductances (Markram and Sakmann, 1994; Seamans et al., 1997). We thus studied the dynamics of IP3-evoked Ca2+release in layer V pyramidal neurons in the prefrontal cortex, using whole-cell electrophysiological recordings combined with video-rate two-photon imaging from neurons filled with fura-2 and caged IP3. Our results show that Ca2+ liberated from IP3-sensitive stores directly affects neuronal excitability and interacts with other Ca2+sources to modulate signaling in cortical neurons.
Materials and Methods
Slice preparation. Brain slices were prepared as described previously (Aghajanian and Marek, 1997) and in adherence with protocols approved by the University of California, Irvine Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering. Briefly, 2.5- to 3.5-week-old FVB/N mice were deeply anesthetized with halothane and decapitated. The brains were quickly removed and placed in ice-cold artificial CSF (aCSF) bubbled with 95%O2–5% CO2. A 300-μm-thick coronal section containing the prefrontal cortex was blocked and glued to the stage of a Vibroslice (Campden Instruments, Loughborough, UK) oscillating microslicer. The standard aCSF used in the recording chambers was composed of the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 10d-glucose, 25 NaHCO3, 2 CaCl2, and 1.2 MgSO4, pH 7.3–7.4. Flow rate for aCSF was ∼3 ml/min. There was at least a 1 hr recovery period before the initiation of experiments. Layer V pyramidal neurons were selected in a region approximately one-half of the distance between the pial surface and the subcortical white matter in the medial prefrontal cortex.
Whole-cell recordings and solutions. Visualized whole-cell patch-clamp recordings were performed using an infrared–differential interference contrast setup. Slices were placed in a custom-made perfusion chamber mounted on a movable stage assembly on an upright microscope (BX50WI; Olympus Optical, Tokyo, Japan). Patch pipettes (4–5 MΩ) were pulled from boroscillicate glass tubing (World Precision Instruments, Sarasota, FL) using a Brown and Flaming horizontal puller (model P-97; Sutter Instruments, Novato, MA) and were filled with a solution containing the following: 135 mmK-methlysulfonate, 10 mm HEPES, 10 mm Na-phosphocreatine, 2 mmMgCl2, 4 mm NaATP, and 0.4 mm NaGTP, pH adjusted to 7.3–7.4 with KOH, as well as 50 μm fura-2 (Molecular Probes, Eugene, OR) and 50 μm caged IP3 (Molecular Probes). Signals were acquired at 1 kHz using an Axopatch 1C amplifier (Axon Instruments, Union City, CA), and analyzed using Clampex 8.1 and Clampfit 8.1 software. Access resistance was continually monitored, and cells were used for recording only if the access resistance was maintained <7 MΩ.
For local agonist stimulation, a puffer pipette filled with 100 μm 1-aminocyclopentane-trans-1S3R-dicarboxylic acid (1S3R-ACPD) (Sigma, St. Louis, MO) was positioned directly over various regions of the cell, including the base and apical regions of the soma, and proximal and distal dendrites. The agonist was ejected using 100 msec pneumatic pressure pulses (PV 800 pneumatic pump; World Precision Instruments).
Ca2+ imaging and flash photolysis. Imaging was performed using a home-made video-rate two-photon microscope, as described previously (Nguyen et al., 2001). In brief, excitation was provided by trains (80 MHz) of ultra-short (∼100 fsec) pulses at 780 nm from a Ti/sapphire laser (Tsunami; Spectra-Physics, Mountain View, CA) pumped by a solid-state laser (Millenia 5X; Spectra-Physics). The laser beam was scanned by a resonant galvanometer (General Scanning Lumonics, Waterton, MA) allowing rapid (7.9 kHz) bidirectional scanning in thex-axis and by a conventional linear galvanometer in they-axis, to provide a frame-scan rate of 30 frames per second. The scanned beam was introduced into the microscope through a custom port mounted between the trinocular head and epifluorescence illuminator and focused onto the specimen through a 40× water-immersion objective (numerical aperture, 0.8). Emitted fluorescence light was collected through the same objective and, after passing through a short-pass filter (λ < 650 nm) to block laser light, was detected by a wide-field photomultiplier (R5929; Hamamatsu, Bridgewater, NJ) mounted on the photo-port of the trinocular head. Luminance and synchronization signals were sampled by a computer equipped with a frame-grabber board (DT3152; Data Translation, Marlborough, MA), and a custom software routine appropriately flipped and interlaced alternate lines of the bidirectional scan to display images (450 × 400 pixels) at 30 Hz. Finally, a video signal derived from the computer display was redigitized by a second computer running the MetaMorph image analysis and processing package (Universal Imaging, Westchester, PA).
All results presented here were obtained using fura-2 as the Ca2+ indicator because, in preliminary trials, we found it to offer advantages over visible-wavelength indicators such as Calcium Green-1. Most significantly, two-photon excitation of fura-2 at 780 nm is equivalent to conventional (one-photon) excitation at 380 nm, in that it shows a decrease in fluorescence on binding Ca2+ rather than an increase as with most other indicators. The basal fluorescence is thus high, facilitating identification of cellular structures and measurement of small Ca2+ increases over the resting level. Second, fura-2 showed a large dynamic range, with the fluorescence intensity changing >10-fold from resting to saturating [Ca2+]. For clarity of presentation, images and traces of fura-2 fluorescence are expressed as a pseudoratio F0/ΔF(where F0 is resting fluorescence and ΔF is the decrease of fluorescence on stimulation), so that increases in [Ca2+] correspond to increasing ratios.
Photolysis of caged IP3 was accomplished by flashes of UV light (340–400 nm) derived from a 100 W Hg arc lamp in a standard Olympus Optical lamphousing and epifluorescence attachment, modified to accept an electronically controlled shutter (Uniblitz, Rochester, NY). The irradiance at the specimen was ∼51 mW mm−2, focused as a uniform circle (radius, ∼50 μm) centered on the imaging field. No attenuation was used in the light path, and the stimulus strength was regulated by the flash duration. On the basis of previous calibration (Parker and Ivorra, 1992), a flash of 10 msec duration would have photolysed ∼4% of the total caged IP3, resulting in an intracellular concentration of free IP3 of ∼2 μm.
Results
IP3-evoked Ca2+release was imaged in layer V pyramidal neurons within the anterior cingulate/infralimbic regions of the medial prefrontal cortex. Morphologically identified pyramidal neurons (n = 106) were selected in an area approximately one-half of the distance between the pial surface and the subcortical white matter and had electrophysiological properties consistent with regularly spiking pyramidal neurons in the neocortex (McCormick et al., 1985), with resting membrane potentials of −63.7 ± 0.4 mV, input resistance of 201.1 ± 6.3 MΩ, and displayed spike frequency adaptation with suprathreshold depolarizing current injections.
Imaging Ca2+ signals evoked by IP3and by action potentials
In this study, we explored the dynamics and functional roles of Ca2+ liberation through IP3 receptor–channels in cortical neurons. Figure 1 illustrates many of the key spatial and temporal Ca2+ characteristics we describe and shows representative Ca2+signals in a neuron that responded strongly to photoreleased IP3 together with signals arising from Ca2+ entry through voltage-gated Ca2+ channels (VGCC) during trains of action potentials.
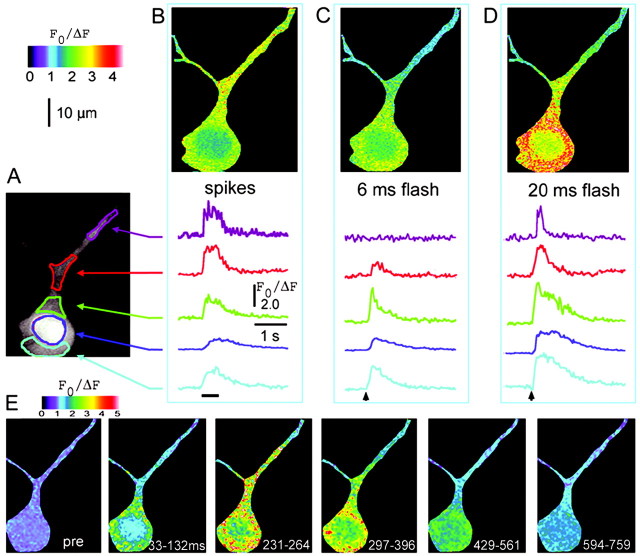
Fig. 1.
Spatial and temporal dynamics of Ca2+ signals evoked in a cortical pyramidal neuron by action potentials and photoreleased IP3.A, Two-photon image showing resting fluorescence of a fura-2-loaded neuron, with regions of interest used to measure Ca2+ signals marked in differentcolors. B, Ca2+transients resulting from a train of action potentials evoked by depolarizing current injection (400 pA for 500 msec; marked bybar). Image shows fluorescence changes during the spike train, expressed as a ratio (F0/ΔF) of the mean resting fluorescence at each pixel (F0) to that at the same pixel during stimulation (ΔF). Increasing ratios (corresponding to increasing [Ca2+] and decreasing fluorescence of fura-2 with 780 nm femtosecond excitation) are depicted as increasingly warmcolors, as denoted by the color bar. The ratio image was formed from four averaged video frames during stimulation and four control frames.Traces show measurements of fura-2 fluorescence ratios from the regions marked in A. C,D, Ca2+ signals imaged in the same neuron in response to photorelease of IP3 by photolysis flashes with respective durations of 6 and 20 msec, delivered when indicated by the arrowheads. E, Image sequence showing the spatial distribution of Ca2+fluorescence signal at different times after the 20 msec photolysis flash. Panels show the mean fluorescence ratio averaged over 66–198 msec (2–6 video frames) before stimulation (pre) and at the specified times (in milliseconds) after the photolysis flash.
Patterns of Ca2+ signaling varied significantly across cellular regions (soma vs dendrite) and by means of activation (photolysis of caged IP3 vs action potentials). Signals evoked by action potentials were relatively small and slow in the soma, whereas larger and more rapidly decaying signals were observed in the dendrites (Fig. 1B). In contrast, large Ca2+ signals were evoked in the soma by brief photolysis flashes (Fig. 1C), whereas in the dendrite the threshold for evoking a response was higher and, in many cells, signals could not be evoked at all. In those cells showing dendritic IP3-evoked Ca2+ responses, the decay rates of dendritic Ca2+ signals were markedly faster than in the soma (Fig 1C,D).
IP3-evoked Ca2+signals in the dendrite did not result simply from passive diffusion of Ca2+ from the soma, because responses to suprathreshold flashes began abruptly after an initial latency, whereas no responses were seen to subthreshold flashes that nevertheless evoked large Ca2+ signals in the soma (Fig.1C–E). We did not observe propagating Ca2+ waves, as have been described in hippocampal neurons (Nakamura et al., 1999); instead, heterogeneous patterns of Ca2+ responses were observed within the soma and proximal dendrite, suggesting that Ca2+ liberation occurs autonomously in discrete subcellular regions. Signals in the nucleus were smaller, and slower, in both rise and decay times than adjacent regions of the cytoplasm, indicating that the nucleoplasm acts merely as a passive sink for Ca2+ diffusion.
Cortical pyramidal neurons show widely varying sensitivities to IP3
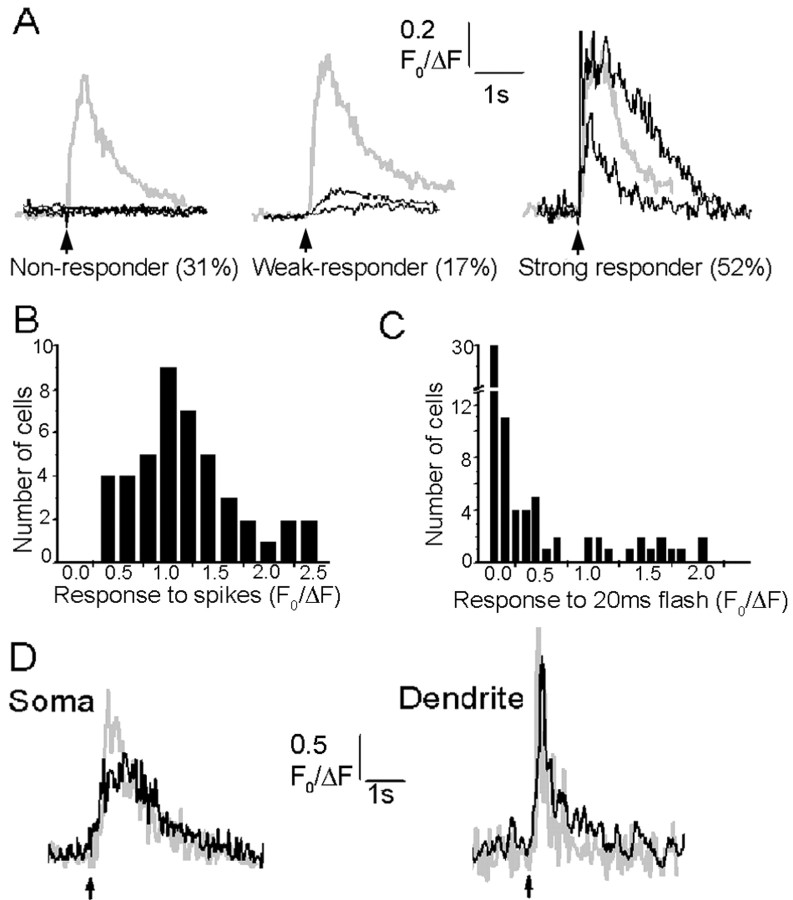
Fluorescence signals evoked by a train of action potentials (0.5 sec depolarizing pulse evoking spikes at ∼20 Hz) were consistently observed in the soma of all neurons, and their amplitudes varied between cells in an approximately Gaussian manner (Fig.2A,B). In contrast, responses to photoreleased IP3varied enormously between cells: many showed strong signals as in Figure 1, but others showed only weak responses or failed to respond at all (Fig. 2A). To quantify these differences, we measured the distribution of fluorescence signals evoked in 72 neurons by UV flashes of fixed (20 msec) duration (Fig. 2C). Many cells showed no detectable response, whereas the remainder exhibited peak fluorescence signals varying continuously over a >10-fold range. For additional analysis, we grouped cells into three categories (Fig.2B): “strong responders” (38%), “weak responders” (21%,) and “nonresponders” (41%). Strongly responding cells showed large, rapidly rising signals in the soma after brief (≤10 msec) photolysis flashes and usually elicited signals in the proximal dendrite with longer flashes. Nonresponders gave no detectable Ca2+ signals in either the soma or dendrites, even with flash durations 10 times greater than those evoking large responses in responding cells. Weakly responding cells showed small, slowly rising Ca2+ signals, usually restricted to the soma, and required longer (30 msec) flashes to evoke threshold responses.
Fig. 2.
Variability in responses to photoreleased IP3. A, Examples from three neurons that typify the three classes of Ca2+ signals evoked among different neurons by photoreleased IP3. Gray traces show responses to trains of action potentials (500 pA depolarizing current for 500 msec), and black traces are responses to photolysis flashes of varying durations. All cells showed large fluorescence signals to spike trains, but nonresponding cells failed to give detectable responses even to long photolysis flashes (left; 20, 100, and 150 msec flashes). Weakly responding cells showed only small, slowly rising signals with long flashes (middle; 10 and 100 msec flashes), whereas even brief photolysis flashes evoked large, rapid signals in strongly responding cells (right; 7 and 30 msec flashes). B,C, Histograms showing, respectively, the distributions of peak somatic fluorescence signals evoked in different neurons (n = 72) by trains of action potentials (7–10 spikes per 500 msec train) and photolysis flashes (20 msec duration). Measurements were obtained as in A. D,Black traces show Ca2+ responses evoked in the soma (left) and proximal dendrite (right) by local application of the group I metabotropic glutamate receptor agonist, 1S3R-ACPD, via a puffer pipette placed near the base of the soma in the same cell.Gray traces show, for comparison, signals evoked by photoreleased IP3. Arrows indicate timing of agonist application (100 msec pressure pulse) and photolysis flashes (20 msec). Similar results were obtained in a total of four neurons, in which the magnitudes and kinetics of agonist-evoked responses matched closely those evoked by photoreleased IP3.
Photolysis flashes that evoked just suprathreshold Ca2+ signals in strongly responding neurons (<10 msec duration, 51 mW mm−2) resulted in intracellular IP3 concentrations of ∼2 μm (see Materials and Methods), whereas concentrations >20 μm failed to give signals in nonresponding cells.
Ca2+ signals mediated via metabotropic receptors
The above experiments used flash photolysis to directly elevate intracellular [IP3] and demonstrate the presence of functional IP3 receptors in cortical pyramidal neurons. To further establish the physiological involvement of these receptors in signaling via Gq-coupled metabotropic receptors, we imaged Ca2+signals evoked by local application of a metabotropic glutamate agonist (1S3R-ACPD) from a puffer pipette placed directly over specific cell regions. Ca2+ signals were evoked only when the pipette was positioned near the basal region of the soma. As illustrated in Figure 2D, metabotropic receptor activation elicited Ca2+ signals in both soma and proximal dendrite that closely matched responses evoked in the same neuron by photoreleased IP3.
Dose–response relationship of IP3-evoked Ca2+ signals in the soma and dendrite
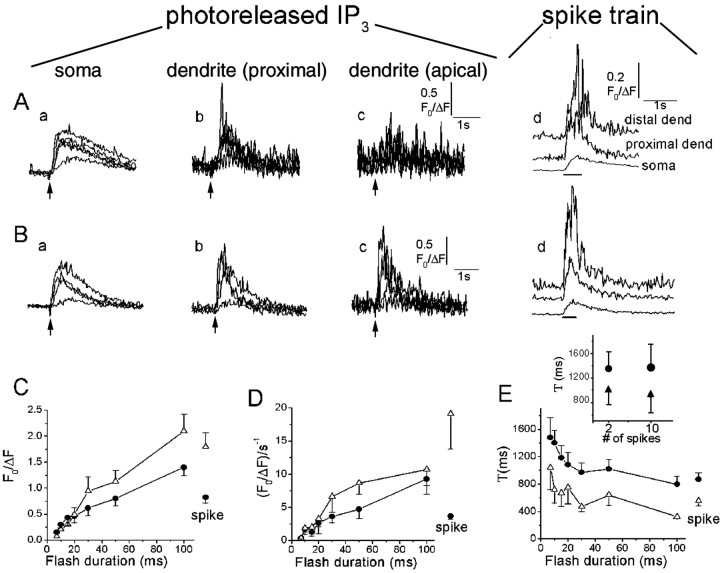
We quantified the magnitude and kinetics of IP3-evoked Ca2+signals evoked by UV flashes in 11 neurons characterized as strong-responders. Figure 3, Aand B, shows representative Ca2+ responses evoked by UV flashes of increasing durations in the soma, apical dendrite, and distal dendrite regions of two cells: the cell in Figure 3B showed responses in the distal dendrite, whereas that in Figure 3A did not. Figure 3, C and D, shows, respectively, the mean peak amplitude and rate of rise of Ca2+signals in the soma and proximal dendrite as functions of UV flash duration. In both the soma and proximal dendrite, the relationships were nonlinear, in that a threshold duration of the photolysis flash was required to evoke detectable Ca2+signals. The amplitude and rate of rise of the Ca2+ signals, however, then increased progressively with longer flash durations.
Fig. 3.
Ca2+ signals evoked by photorelease of increasing amounts of IP3.A, Records from a strongly responding neuron, illustrating different spatial patterns of Ca2+signals evoked by photorelease of increasing amounts of IP3. Superimposed traces in the first three panels show fluorescence signals recorded from the soma (a; excluding nucleus), proximal dendrite (b; within 10 μm of the soma), and distal dendrite (c; 50 μm from soma), in response to photolysis flashes with durations of 7, 10, 15, and 20 msec. The right panel (d) shows corresponding responses in each region to trains of action potentials. B, Similar records from another neuron, in which large responses were observed in the distal dendrite. Flash durations were 7, 10, 20, and 50 msec. C, Mean peak amplitude of fluorescence signals in the soma (filled circles) and proximal dendrite (open triangles) of 11 strongly responding neurons, plotted as a function of photolysis flash duration. Amplitudes of signals evoked by spike trains in the same neurons are shown at theright. Error bars indicate 1 SEM. D, Corresponding measurements of rate of rise (F0/ΔFsec−1) of the fluorescence signals. Only suprathreshold Ca2+ responses were included in calculating the averaged values. E, Time constants of decay of fluorescence signals in the same neurons, derived from single-exponential fits to the decay phase of the IP3-evoked Ca2+ responses.Inset shows the Ca2+ decay time constants for a single spike and a train of 10 spikes, measured in both the soma and dendrite of a separate population of neurons (n = 5).
The rates of decay of both IP3-evoked and spike-evoked Ca2+ signals were markedly faster in the dendrites compared with the soma (Fig.3A,B,E), indicating a faster rate of dendritic Ca2+ clearance that may arise as a result of the differing geometries (ratio of surface area to volume) of these regions. Interestingly, the decay of IP3-evoked Ca2+signals became more rapid with progressively stronger stimuli, although their peak amplitude increased. This change in decay rate was not observed for Ca2+ signals evoked by spike trains of varying durations (Fig. 3E, inset), indicating that it does not arise through varying rates of Ca2+ clearance at differing intracellular Ca2+ concentrations. Instead, a likely mechanism is that Ca2+ signals at low IP3 concentrations are prolonged because of sustained liberation of Ca2+ from intracellular stores, whereas liberation at high [IP3] is more transient, because of either rapid inactivation of release channels or depletion of intracellular stores.
Responses to paired photolysis flashes
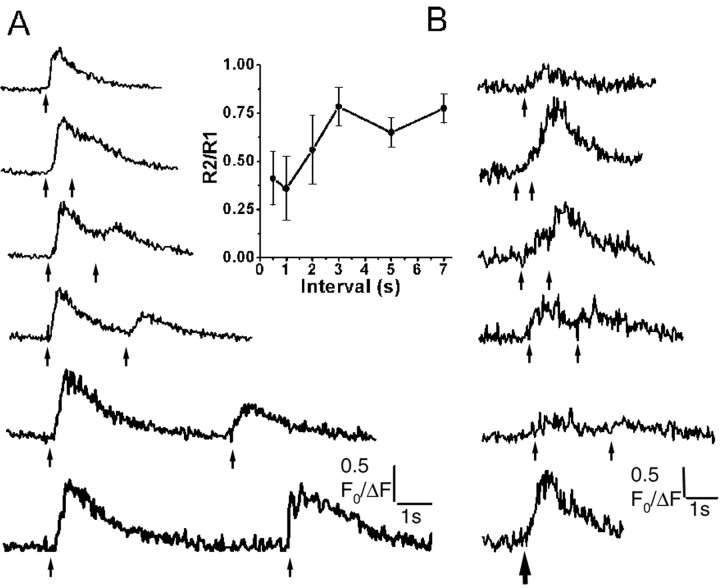
In nonexcitable cells, Ca2+liberation evoked by photoreleased IP3 is depressed for several seconds after a preceding suprathreshold UV light flash (Parker and Ivorra, 1993), probably as a result of feedback inhibition by Ca2+ on the IP3R (Bezprozvanny et al., 1991; Finch et al., 1991). To examine whether a similar phenomenon occurs in pyramidal neurons, we delivered paired, identical flashes at varying intervals in strongly responding cells. As illustrated in Figure4A, the Ca2+ signal evoked by a second, suprathreshold flash was strongly reduced at an interval of 1 sec but subsequently recovered over several seconds. These effects cannot be attributed to dye saturation or consumption of caged IP3, because flashes of twice the test duration produced appreciably larger signals than each individual flash.
Fig. 4.
Inhibition and summation of IP3-evoked Ca2+ release with paired-flash protocols. A, Fluorescence signals in the soma of a strongly responding neuron evoked by paired photolysis flashes (both 50 msec duration) delivered at varying intervals, as indicated by the arrows. Inset plots the size of the second response relative to the first response in each pair, as a function of interflash interval. Data are mean ± 1 SEM from six neurons. B, Data from a different neuron, in which additive responses were observed with pairs of brief UV flashes (15 msec) at short intervals.
In contrast to the paired-flash inhibition seen with suprathreshold stimuli, Ca2+ liberation inXenopus oocytes is greatly potentiated with paired flashes that are individually too weak to evoke signals (Parker and Ivorra, 1992), suggesting that a chemical summation of IP3 levels might serve as the basis for a novel form of synaptic facilitation (Parker and Miledi, 1989). However, experiments in cortical neurons (n = 6), using paired photolysis flashes that were individually near the threshold required to evoke detectable somatic Ca2+ signals, failed to reveal any marked potentiation of responses to the second flash. Most cells showed a suppression, as in Figure4A, but one neuron displayed an additive effect with photolysis flashes at intervals <2 sec (Fig. 4B).
Bidirectional interactions between IP3-evoked Ca2+ release and extracellular Ca2+ influx
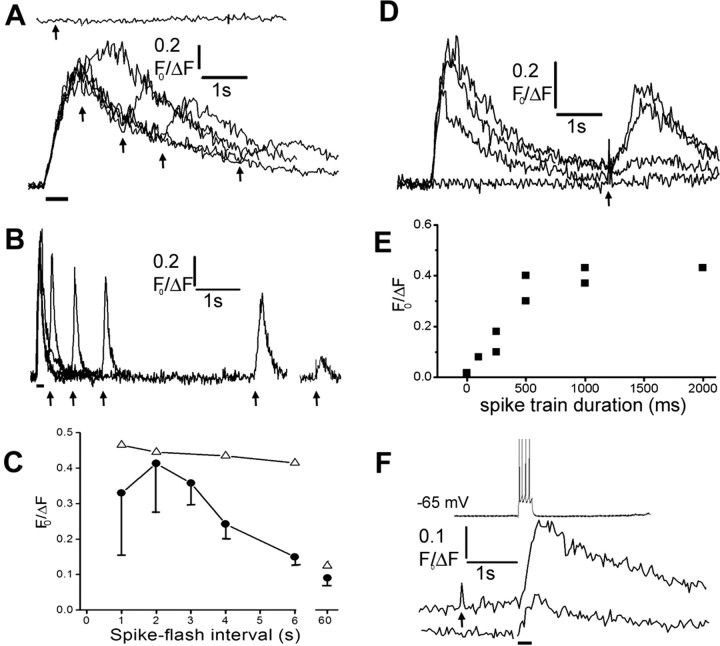
To study the interactions between Ca2+ signals arising from extracellular and intracellular sources, we first examined the effects of Ca2+ influx during action potentials on subsequent Ca2+ liberation evoked by photorelease of IP3 (“spike-flash” paradigm). In all strongly responding neurons examined (n = 9), IP3-evoked Ca2+signals were unaffected by a preceding train of action potentials. However, in many weak-responding or nonresponding neurons, a preceding train of action potentials (∼18 Hz, 0.5 sec) strongly potentiated or “rescued” IP3-evoked Ca2+ signals (11 of 21 cells tested) (Fig.5A,B,D). The rescued neurons displayed two different temporal patterns of potentiation, suggesting different underlying mechanisms. Most (9 of 11) displayed a short-lasting potentiation of the IP3 response (Fig. 5A, filled symbols in C), which was maximal near the peak of the spike-evoked Ca2+ signal and decayed by 50% within ∼4 sec. This decline approximately mirrored the fall in cytosolic [Ca2+] after the spike train, suggesting that Ca2+ liberation may have been potentiated by a facilitatory action of Ca2+ on the IP3receptor. In contrast, two other neurons displayed a prolonged potentiation that remained evident for tens of seconds after the spike-evoked Ca2+ signal had decayed to baseline (Fig. 5B, open symbols in C) and was strongly dependent on the duration of the preceding spike train (Fig. 5D,E,). These properties appear consistent with a mechanism by which Ca2+ influx during action potentials results in increased filling of leaky IP3-sensitive stores.
Fig. 5.
Reciprocal facilitation between Ca2+ signals arising from extracellular influx and liberation from IP3-sensitive stores. A, Transient rescue of IP3 response in a nonresponding neuron after Ca2+ entry during trains of action potentials. The top trace shows the fluorescence signal evoked by a photolysis flash (arrow; 100 msec duration) alone. Thebottom superimposed traces show responses to photolysis flashes of the same duration delivered at varying times (arrows) after a spike train (bar; 500 msec, 500pA). B, Longer-lasting facilitation in a different neuron. Superimposed traces show responses to photolysis flashes of fixed duration (100 msec) delivered at varying times (marked by arrows) after a spike train (indicated by bar; 500 msec, 500 pA). The rightmostresponse was evoked by a flash applied 60 sec after the spike train.C, Time course of decay of facilitation of IP3-evoked response as a function of the interval after preceding spike trains. Filled symbols are mean measurements with SEM from nine neurons like that in A, in which facilitation decayed rapidly. Open trianglesare means from two neurons displaying a more sustained facilitation, as in B. D, The facilitation of IP3-evoked Ca2+ release increases with increasing duration of a preceding spike train. Superimposed traces show fluorescence signals evoked by constant photolysis flashes (50 msec duration) delivered at a fixed interval (4 sec;arrow) after spike trains with durations of 0, 250, 500, and 1000 msec. E, Mean peak amplitude of IP3-evoked fluorescence signal measured in two neurons as a function of duration of a preceding spike train from records like those in D. F, Ca2+ signals evoked by a brief train of action potentials are facilitated after photorelease of IP3. Traces show (fromtop to bottom) a train of four action potentials and Ca2+ signals evoked by the action potential train with and without a photolysis flash (200 msec duration; delivered at arrow).
We then looked for the inverse interaction, by using a flash-spike paradigm in which action potentials were evoked by injecting depolarizing current pulses after photolysis flashes. In three neurons examined, we observed a consistent potentiation, in which submaximal Ca2+ signals evoked by a few action potentials (∼4 spikes) were greatly enhanced (272 ± 41.1% of control; n = 7 trials) when preceded by photolysis flashes that, themselves, failed to evoke strong responses (Fig.5F).
IP3-evoked Ca2+ release reduces membrane excitability
Cortical pyramidal neurons exhibit a characteristic spike frequency adaptation, whereby Ca2+ entry during action potentials leads to activation of a Ca2+-dependent outward K+ current (IAHP) that, in turn, reduces membrane excitability and increases the interspike interval (McCormick et al., 1985; Sah, 1996). We examined whether Ca2+liberated from IP3-sensitive intracellular stores could similarly modulate spiking patterns.
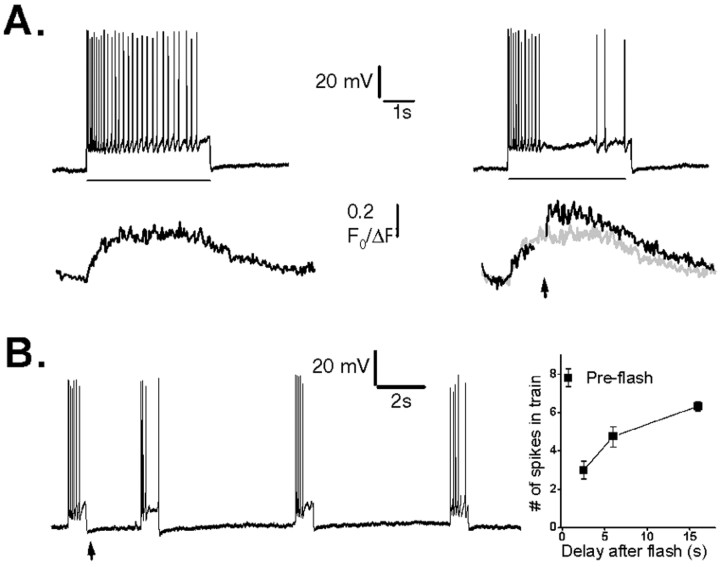
Trains of spikes were induced by injecting depolarizing currents (300–500 pA, 0.5–2 sec) through the patch pipette in current-clamp mode. Photorelease of IP3 in strongly responding cells during these spike trains resulted in an incremental increase in Ca2+ signal and a strong suppression of action potentials (n = 6) (representative example shown in Fig. 6A). To measure the duration for which photoreleased IP3 could depress neuronal excitability, we applied brief depolarizing current pulses of constant amplitude and duration at varying times after a photolysis flash and counted the number of spikes evoked during each pulse (Fig. 6B). Spike frequency was depressed to approximately one-third of the control level 2 sec after photorelease of IP3 and had not fully recovered even after 16 sec (Fig. 6C).
Fig. 6.
Suppression of action potential spiking by photorelease of IP3. A, Top traces show action potentials evoked by fixed depolarizing current pulses (200 pA, 4 sec; bar), and bottom traces show corresponding Ca2+ fluorescence signals recorded in the soma. A photolysis flash was delivered when marked by the arrow in the records on theright, resulting in a transient suppression of spikes and a larger Ca2+ signal (black trace) than evoked by the spike train alone (superimposed gray trace). B, Sustained depression of membrane excitability after photorelease of IP3. The neuron was stimulated by repeated depolarizing current pulses (120 pA, 800 msec), and a photolysis flash was delivered when indicated by the arrow. C, Mean measurements from 10 neurons, showing the number of action potentials generated during fixed depolarizing pulses delivered at varying intervals after a UV flash. The experimental protocol was the same as in B.
IP3-evoked Ca2+ release activates an outward membrane current
The depression of spike firing after photorelease of IP3 likely involves activation of a Ca2+-dependent outward current. In agreement, photolysis flashes evoked small hyperpolarizations of graded amplitude from a resting membrane potential of approximately −60 mV, with time courses approximately paralleling accompanying Ca2+ signals recorded in the soma (Fig.7A). Furthermore, voltage-clamp experiments (n = 10; holding potential, −60mV) demonstrated the activation of a transient outward current, the peak amplitude of which was graded with the flash duration (Fig.7B) and increased after an approximately second-power relationship with the peak of the corresponding Ca2+ signal (Fig. 7C).
Fig. 7.
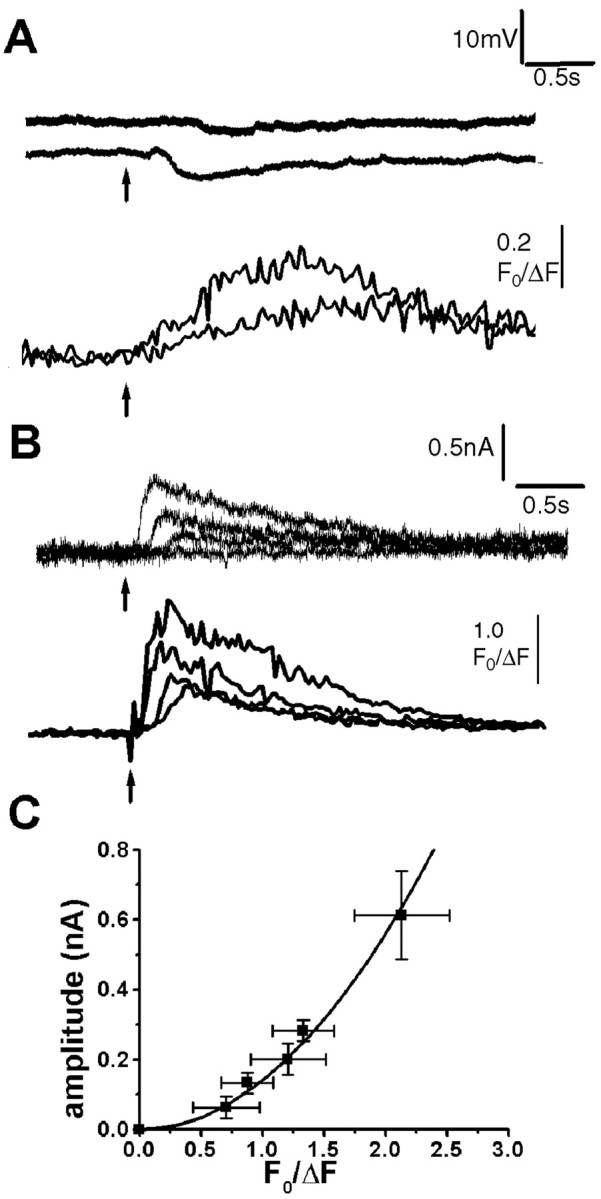
Photorelease of IP3 produces membrane hyperpolarization and associated outward membrane currents, proportional to the magnitude of the IP3-evoked Ca2+ signal. A, Changes in membrane potential (top traces) and fluorescence Ca2+ signal (bottom traces) evoked by photolysis flashes with durations of 20 and 100 msec. Resting potential was −60 mV. B, Outward membrane currents and fluorescence signals evoked in a voltage-clamped neuron at a potential of −60 mV by photolysis flashes with durations of 25, 50, 75, and100 msec. C, Relationship between peak amplitude of the IP3-evoked Ca2+ signal and the corresponding peak outward membrane current, measured from voltage-clamp experiments in nine neurons. Curve is a second-power relationship fitted to the data.
Discussion
Although neurons in the prefrontal cortex express a high density of IP3Rs and use calcium signaling for mediating complex cognitive and behavioral functions, surprisingly little is known regarding IP3 signaling in cortical neurons. We therefore undertook these studies to elucidate the basic properties of the IP3–Ca2+ signaling pathway in layer V pyramidal cortical neurons.
Variability in IP3-mediated Ca2+ signals
A striking initial finding was the wide variability in IP3-mediated Ca2+signals between individual neurons. Although all pyramidal cells showed strong Ca2+ signals during trains of action potentials, some gave large Ca2+responses with relatively weak photorelease of IP3, whereas others failed to respond to even strong stimuli. This variability does not appear to arise through developmental differences between animals (Yamamoto et al., 2002), because slices were obtained from relatively mature mice (2.5–3.5 weeks old) and, most significantly, responding and nonresponding cells were found within the same slice. Instead, several mechanisms may contribute to the varying IP3 responses. One simply involves the numbers and type of IP3Rs present in individual cells. The density and distribution of the IP3Rs and IP3R subtypes vary within the population of pyramidal cells (Worley et al., 1989;Sharp et al., 1993, 1999), and both the maximal response and threshold sensitivity to IP3 are expected to depend on IP3R density (Nakamura et al., 2002). A second putative mechanism involves differential modulation of IP3R function between cells. Finally, IP3-evoked Ca2+liberation depends on the state of ER Ca2+stores, reflecting previous activity history and serving as an “activity memory bank” (Simpson et al., 1995; Garaschuk et al., 1997). The rescue of IP3 responses in apparently weak-responding and nonresponding cells by Ca2+ influx during tetanic stimulation suggests that store filling may be a major regulatory mechanism.
These experiments were performed without blocking RyR, so it is possible that Ca2+ signals may have been amplified by subsequent CICR through RyR. Nevertheless, by virtue of the use of photoreleased IP3, it is clear that the primary signal reflects activation of IP3R.
Spatial and temporal aspects of Ca2+ signals
Photolysis of caged IP3 is expected to result in a uniform elevation of intracellular [IP3] throughout the irradiated region of the cell. The observed spatial gradient in responses to photoreleased IP3 (with the soma showing the lowest threshold for IP3 and progressively higher thresholds or loss or response through the distal dendrites) thus reflects differential sensitivity to IP3 that may result from the higher density of IP3 receptors in the soma and proximal dendrites in these neurons (Sharp et al., 1999). In contrast, Ca2+ signals generated by action potentials were larger in the dendrites, consistent with the distribution of VGCCs (Seamans et al., 1997), and the lower ratio of cytosolic volume per unit area of plasma membrane in the dendrites. The decay of Ca2+ signals evoked both by IP3 and action potentials was consistently faster in the dendrites than the soma, again likely attributable to the higher surface-to-volume ratio in dendrites. Interestingly, in all subcellular regions, the decay of IP3-evoked Ca2+ signals became faster with increasingly strong stimuli and approached that of signals evoked by trains of action potentials. A likely explanation is that the decline of cytosolic [Ca2+] is ultimately limited by clearance mechanisms but that IP3-evoked responses are further prolonged by continued liberation through IP3R. Stronger activation may result in more rapid termination of Ca2+ liberation, either through Ca2+ inhibition of IP3R or simply because the ER Ca2+ stores empty faster.
Ca2+ liberation in pyramidal cells showed a nonlinear dose dependence at low [IP3], in that a certain threshold flash duration was required to evoke detectable signals. A similar nonlinearity has been described in nonexcitable cells (Parker and Ivorra, 1992; Bootman et al., 1997) and in Purkinje cells (Khodakhah and Ogden, 1993) and arises through the threshold sensitization needed to induce regenerative CICR (Bootman et al., 1997; Marchant et al., 1999). This nonlinearity could provide a basis for “chemical integration” of IP3signals, leading to facilitation of successive Ca2+ responses; however, we were unable to find convincing evidence for this phenomenon in pyramidal neurons. Instead, responses to paired photolysis flashes showed at most only an additive effect with weak stimuli, whereas the Ca2+ response to an initial strong flash resulted in a marked suppression of the response to a second flash.
Interactions between extracellular and intracellular Ca2+ sources
The two Ca2+ sources for generating cytosolic Ca2+ signals are the intracellular stores and the larger reservoir of extracellular Ca2+. Interactions between these pathways are expected to be complex, because of both the biphasic facilitatory–inhibitory actions of Ca2+on the IP3 receptor and because the extent of store filling varies with the history of cytosolic Ca2+ transients (Simpson et al., 1995;Berridge, 1998).
Our principal finding was a bidirectional facilitation between Ca2+ influx through VGCCs and Ca2+ liberation through IP3Rs. Thus, spike trains evoked before photorelease of IP3 in a weak-responding or nonresponding neuron could rescue an IP3-evoked Ca2+ response, essentially converting that neuron into a strong responder. The extent of facilitation increased with increasing amount of Ca2+ influx through VGCCs and, in different neurons, decayed with fast (a few seconds) or slow (tens of seconds) time courses. These two kinetically different responses may reflect different underlying mechanisms, respectively, a direct agonist effect of Ca2+ on the IP3R and enhanced filling of ER Ca2+ stores. Ca2+ influx through VGCCs produced little or no facilitation in cells that already showed strong responses to IP3, but it is possible that the sensitivity of IP3Rs and extent of store filling were already near maximal. Conversely, previous photorelease of IP3 potentiated Ca2+signals during brief trains of action potentials, probably because Ca2+ entry through VGCCs was amplified by subsequent CICR.
Consistent with our findings, metabotropic receptor activation enhanced Ca2+ responses mediated through VGCCs in hippocampus (Nakamura et al., 1999) and visual cortex (Yamamoto et al., 2002). However, in Purkinje cells, Ca2+influx suppressed subsequent liberation through IP3Rs (Khodakhah and Ogden, 1993). The difference likely lies in a different balance between facilitatory and inhibitory effects of Ca2+. For example, closer proximity of VGCCs to IP3Rs in the dendrites of Purkinje cells might result in a higher local [Ca2+], favoring binding to the inhibitory Ca2+ site of the IP3Rs.
IP3-evoked Ca2+ release reduces membrane excitability
Photorelease of IP3 during a train of evoked action potentials resulted in a transient increase in Ca2+ levels, reduction in spike frequency, and membrane hyperpolarization. The effect on action potential generation mimics the spike frequency adaptation characteristic of cortical pyramidal neurons, in which membrane excitability is reduced by activation of the Ca2+-dependent outward potassium current, IAHP (Sah, 1996). It is likely that Ca2+ released from ER stores similarly activates K+channels, and, in agreement, voltage-clamp recordings revealed a transient outward membrane current after photorelease of IP3, the amplitude of which varied as the second power of the associated Ca2+ signal. The depression of spike activity after photorelease of IP3 persisted as long as 15 sec and considerably outlasted the Ca2+ transient and associated hyperpolarization directly evoked by the flash. The decrease in membrane excitability cannot, therefore, be explained simply by a sustained Ca2+ activation of K+ conductance. Instead, its prolonged time course may mirror the decline of cytosolic [IP3], which enables CICR so that Ca2+ entry during a spike is amplified and thereby tends to inhibit subsequent spike firing.
Physiological roles of IP3–Ca2+signaling in cortical pyramidal neurons
By using caged IP3, we were able to precisely regulate the relative levels and timing of IP3 concentration changes in cortical neurons and thereby examine the specific effects of IP3signaling while circumventing the contributions of upstream second-messenger components that arise via activation of membrane Gq-coupled receptors (Nakamura et al., 1999;Yamamoto et al., 2002). Furthermore, Ca2+responses evoked by photoreleased IP3 resembled those induced by local application of the metabotropic glutamate agonist 1S3R-ACPD, indicating that they mimic physiological signals.
In contrast to the dendritic localization of IP3signaling in cerebellar Purkinje cells, the soma appears to be the dominant compartment for IP3-mediated Ca2+ signaling in cortical pyramidal neurons. Local somatic applications of metabotropic agonist demonstrate a functional input via neurotransmitter receptors, and one important output is the modulation of electrical excitability through Ca2+-activated K+ channels. Furthermore, the strong IP3-evoked Ca2+signals in perinuclear regions raise the possibility for activation of transcription factors and immediate early genes.
Layer V pyramidal neurons serve as crucial integrators of neuronal circuits involved in many cognitive executive functions (Goldman-Rakic, 1995; Birrell and Brown, 2000). The IP3-mediated depression of spiking patterns may directly affect output signals to downstream effector regions. Phosphoinositide-linked inputs to the soma and proximal dendrites of pyramidal neurons are thus likely to serve as powerful and sustained modulators of excitability, acting in a complex, use-dependent manner via reciprocal interactions between electrical and chemical signals.
Footnotes
This work was supported by National Institutes of Health Grants GM48071 (I.P.) and AG16573 (F.M.F.). G.E.S. is supported by National Institute on Aging Training Grant AG00096. We thank Jonathon Marchant, Candace Y. Hsieh, and Raju Metherate for technical assistance and intellectual support.
Correspondence should be addressed to Grace E. Stutzmann at the above address. E-mail: grace@uci.edu.
References
- 1.Aghajanian G, Marek G. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M. Elementary and global aspects of calcium signaling. J Physiol (Lond) 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge M. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M, Lipp P, Bootman M. The versatility and universality of calcium signaling. Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 5.Bezprozvanny I, Watras J, Ehrlich B. Bell-shaped calcium-response curves of Ins(1, 4, 5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 6.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;2020:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global calcium signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 8.Finch E, Turner T, Goldin S. Calcium as a coagonist of inositol 1, 4, 5-triphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 9.Friel D, Tsien R. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol (Lond) 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii S, Matsumoto M, Igarashi K, Kato H, Mikoshiba K. Synaptic plasticity in hippocampal CA1 neurons of mice lacking type 1 inositol-1, 4, 5-trisphosphate receptors. Learn Mem. 2000;7:312–320. doi: 10.1101/lm.34100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol (Lond) 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman-Rakic P. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 13.Iino M. Biphasic Ca2+-dependence of inositol 1, 4, 5-triphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caici. J Gen Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilyin V, Parker I. Role of cytosolic Ca2+ in inhibition of InsP3-evoked Ca2+ release in Xenopus oocytes. J Physiol (Lond) 1994;477:503–509. doi: 10.1113/jphysiol.1994.sp020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci USA. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey A. Immunological localization of m1–m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 17.Marchant J, Callamaras N, Parker I. Initiation of IP(3)-mediated Ca2+ waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA. 1994;2491:5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick DA, Conners BW, Lighthall JW, Prince DA. Comparitive electrophysiology of pyramidal and sparsely spiny neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- 20.Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog Neurobiol. 2001;65:309–338. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 21.Mellstrom B, Naranjo J. Mechanisms of Ca2+-dependent transcription. Curr Opin Neurobiol. 2001;11:312–319. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 22.Miyata M, Finch E, Khiroug L, Hashimoto K, Hayasaka S, Oda S, Inouye M, Takagishi Y, Augustine G, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 23. Morikawa H, Imani F, Khodakhah K, Williams J. Inositol 1, 4, 5-triphosphate-evoked responses in midbrain dopamine neurons. J Neurosci 20 2000. RC103(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Barbara J-G, Nakamura K, Ross W. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Lasser-Ross N, Nakamura K, Ross WN. Spatial segregation and interaction of calcium signalling mechanisms in rat hippocampal CA1 pyramidal neurons. J Physiol (Lond) 2002;543:465–480. doi: 10.1113/jphysiol.2002.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen Q, Callamaras N, Parker I. Consruction of a two-photon microscope for video-rate Ca2+ imaging. Cell Calcium. 2001;30:383–393. doi: 10.1054/ceca.2001.0246. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama M, Hong K, Mikoshiba K, Poo M, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;40:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 28.Parker I, Ivorra I. Characteristics of membrane currents evoked by photoreleased inositol triphoshpate in Xenopus oocytes. Am J Physiol. 1992;263:C154–C165. doi: 10.1152/ajpcell.1992.263.1.C154. [DOI] [PubMed] [Google Scholar]
- 29.Parker I, Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989;9:4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose C, Konnerth A. Stores not just for storage: Intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 31.Sabatini B, Maravall M, Svoboda K. Ca2+ signaling in dendritic spines. Curr Op Neurobiol. 2001;11:349–356. doi: 10.1016/s0959-4388(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 32.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 33.Seamans J, Gorelova N, Yang C. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical neurons. J Neurosci. 1997;17:5936–5948. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp A, McPherson P, Dawson T, Aoki C, Campbell K, Snyder S. Differential immunohistochemical localization of inositol 1, 4, 5 triphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharp A, Nucifora FJ, Blondel O, Sheppard C, Zhang C, Snyder S, Russell J, Ryugo D, Ross C. Differential cellular expression of isoforms of inositol 1, 4, 5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- 36.Simpson PB, Challiss RA, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- 37.Wilson K, Minneman K. Regional variations in alpha 1-adrenergic receptor subtypes in rat brain. J Neurochem. 1989;53:1782–1786. doi: 10.1111/j.1471-4159.1989.tb09243.x. [DOI] [PubMed] [Google Scholar]
- 38.Worley P, Baraban J, Snyder S. Inositol 1, 4, 5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989;9(1):339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K, Hashimoto K, Nakano M, Shimohama S, Kato N. A distinct form of calcium release down-regulates membrane excitability in neocortical pyramidal cells. Neuroscience. 2002;109:665–676. doi: 10.1016/s0306-4522(01)00486-9. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Parker I. Potentiation of inositol triphosphate-induced Ca2+ mobilization in Xenopus oocytes by cytosolic Ca2+. J Physiol (Lond) 1992;458:319–338. doi: 10.1113/jphysiol.1992.sp019420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol triphosphate in Xenopus oocytes. J Physiol (Lond) 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]