Fig. 4.
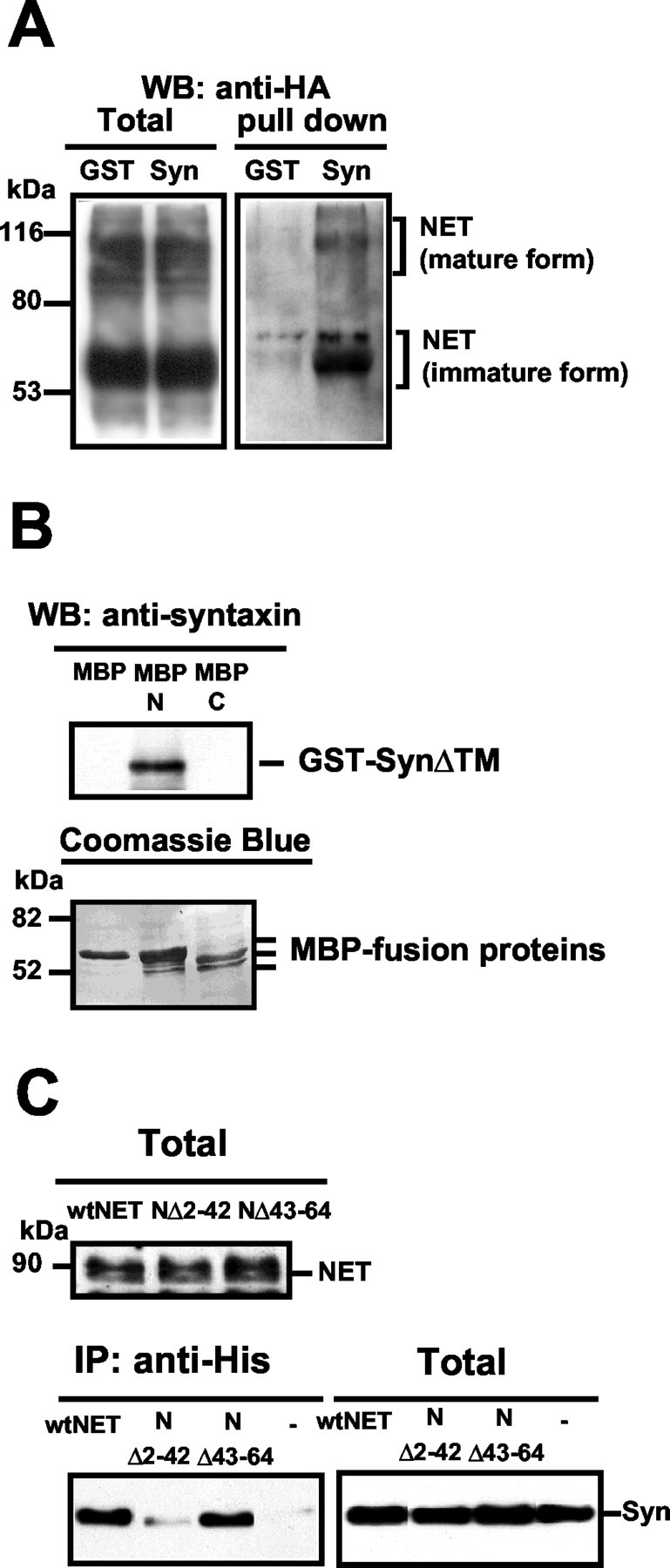
Syntaxin 1A binds NET directly via sequences in the NH2 terminus of the transporter. A, GST-SynΔTM pull-down of NET protein. COS-7 cells were transfected with HA-tagged hNET, and detergent lysates were incubated with glutathione beads precoated with either GST (GST) or GST-SynΔTM (Syn). Proteins bound to the beads were eluted and subjected to SDS-PAGE, followed by immunoblotting with anti-HA. Unlike GST beads, GST-SynΔTM beads retrieved NET proteins both in the immature and mature forms. B, Direct binding of syntaxin 1A cytoplasmic domain to the hNET NH2 terminus. Amylose resins, precoated with equimolar MBP, MBP-hNET NH2 terminal protein (MBP-N), or MBP-hNET COOH terminal protein (MBP-C), were incubated with GST-SynΔTM as described in Materials and Methods, followed by elution of bound material, SDS-PAGE, and immunoblotting for syntaxin 1A. Only MBP-N retained GST-SynΔTM. Membranes subsequently were stained with Coomassie brilliant blue to reveal equivalent amounts of MBP fusion proteins used in the experiments. C, An hNET NH2 terminal deletion disrupts NET/syntaxin 1A coimmunoprecipitation. The top panel shows an immunoblot of hNET along with the NH2 terminal deletion mutants that were used. CHO cells were transfected with either His-hNET (wtNET) or His-hNET mutants NΔ2–42 or NΔ43–64. Aliquots of extracts were analyzed in SDS-PAGE and probed with polyclonal anti-His antibody to reveal equivalent expression. The bottom panel shows results of cotransfection of N or NET mutants (670 ng) with syntaxin 1A (45 ng)/coimmunoprecipitation experiments, immunoprecipitating with anti-His and probing for syntaxin 1A. The NΔ2–42 mutant significantly diminished syntaxin 1A recovery relative to wt hNET or NΔ43–64. Syntaxin 1A expression was equivalent in all samples as assessed with syntaxin 1A immunoblots of total cell extracts. Results presented in A–C are representative of three to six experiments for each condition.
