Fig. 6.
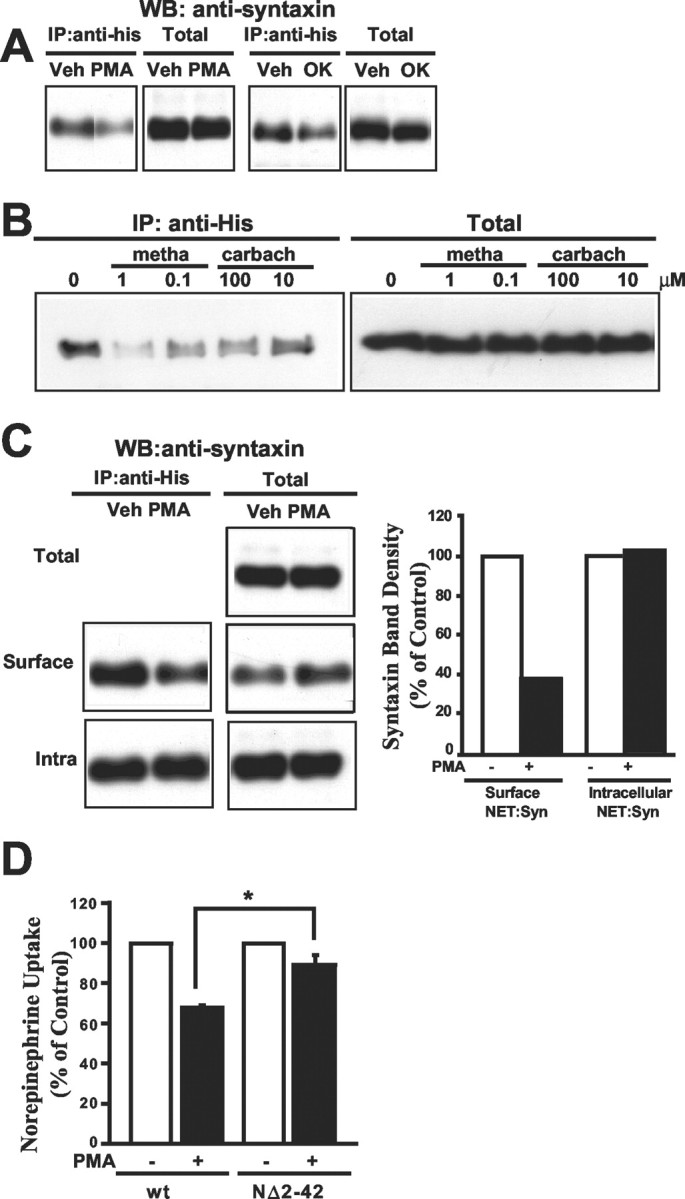
Regulated association of NET and syntaxin 1A in cotransfected CHO cells. A, Phorbol ester or okadaic acid treatments diminish recovery of syntaxin 1A from NET immunoprecipitations. CHO cells, cotransfected with His-hNET and syntaxin 1A, were preincubated with DMSO (Veh), 1 μm β-PMA (PMA), or 1 μmokadaic acid (OK) for 30 min at 37°C before immunoprecipitation with anti-His, SDS-PAGE, and immunoblotting for syntaxin 1A. Total cell extracts for each condition were blotted for syntaxin 1A in parallel. Both PMA and okadaic acid diminished recovery of syntaxin 1A relative to vehicle-treated samples. B, Muscarinic receptor activation diminishes recovery of syntaxin 1A from NET immunoprecipitates. Stable M3 muscarinic receptor-transfected CHO cells that had been cotransfected transiently with His-hNET and syntaxin 1A were treated with the indicated concentrations (in μm) of the muscarinic agonists methacholine or carbachol (30 min) before extraction and immunoprecipitation of complexes with anti-His, SDS-PAGE, and blotting for syntaxin 1A. In parallel, syntaxin 1A was blotted from total cell extracts and is evident at equivalent levels in all conditions. C, Phorbol ester regulation of the interaction between NET and syntaxin 1A occurs with plasma membrane-localized complexes. CHO cells, cotransfected with His-hNET and syntaxin 1A, were treated with DMSO (Veh) or 1 μm β-PMA for 30 min at 37°C. Surface proteins were labeled with NHS-sulfo-biotin at 4°C before cell lysis and recovery of surface complexes (Surface) on avidin beads. Bound proteins were eluted with 2 mm biotin, immunoprecipitated with anti-His, resolved on SDS-PAGE, and immunoblotted for syntaxin 1A. Nonbound (Intra) extracts were immunoprecipitated and blotted in parallel. Phorbol ester-induced reduction in syntaxin 1A in NET immunoprecipitates is evident in surface fractions, but not in intracellular complexes. Blots of total cell extracts (Total) and nonbiotinylated, intracellular samples (Intra Total) show no impact of phorbol ester on syntaxin 1A content. PMA increased syntaxin 1A contents in total biotinylated pools (Surface Total). The bar graph on the right is a quantitation of syntaxin 1A recovery in the immunoprecipitates. D, hNET NH2 terminal deletion that disrupts NET/syntaxin 1A interactions diminishes phorbol ester-mediated NET downregulation. CHO cells were transfected with His-hNET (wt) or hNET NΔ2–42 as described in Figure 4, followed by treatment of cells with 1 μm β-PMA or vehicle for 30 min. Cells receiving hNET NΔ2–42 were significantly less sensitive to phorbol ester treatment with respect to NE transport activity (n = 3; *p < 0.05; Student's ttest).
