Fig. 4.
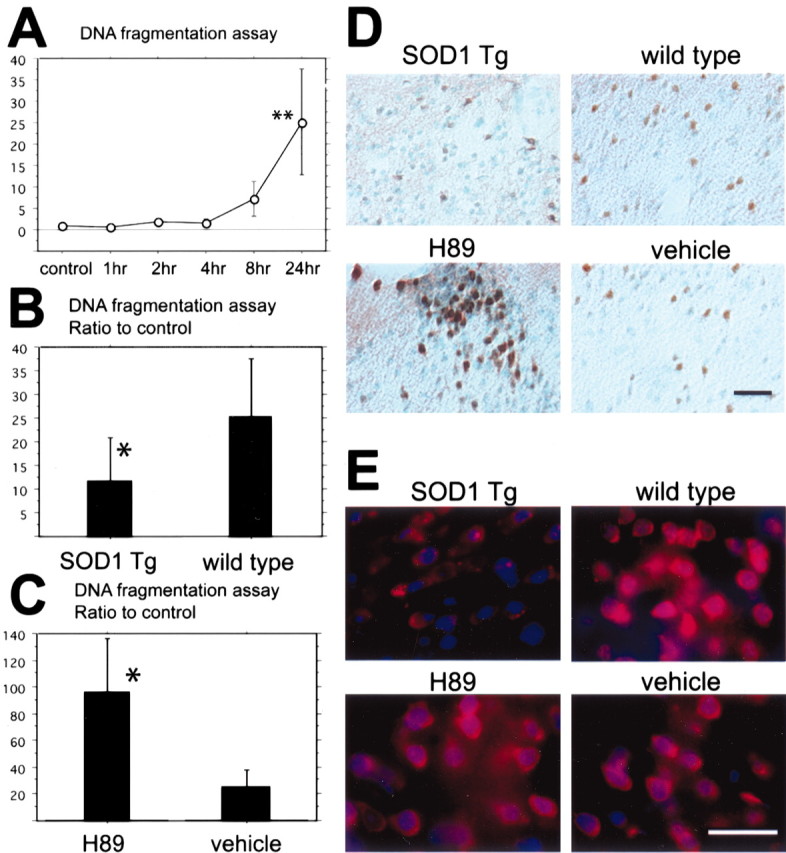
A, Apoptotic-related DNA fragmentation after tFCI was analyzed with a commercial cell death detection kit (n = 4 each). DNA fragmentation increased significantly in the entire MCA territory lesion 24 hr after tFCI (**p < 0.01). B, In the SOD1 Tg mice DNA fragmentation significantly decreased compared with the wild-type mice 24 hr after tFCI (*p < 0.05;n = 4 each). C, In the H89-treated mice, as a protein kinase A inhibitor sample, DNA fragmentation significantly increased compared with the vehicle-treated mice 24 hr after tFCI (*p < 0.05; n = 4 each). D, Representative photomicrographs show TUNEL staining in SOD1 Tg mice (top left panel) and in the H89-treated mice (bottom left panel) and, as a control, in the wild-type mice (top right panel) and the vehicle-treated mice (bottom right panel) 24 hr after tFCI (n = 4 each). TUNEL-positive cells were observed in the ischemic cortex of the MCA territory and the partial caudate–putamen surrounding the ischemic core 24 hr after tFCI. TUNEL reactivity was strongly observed in the wild-type mice (top right panel), whereas in the SOD1 Tg mice TUNEL reactivity decreased (top left panel). TUNEL reactivity in the H89-treated mice was much stronger compared with the vehicle-treated mice (bottom panels). Scale bar, 50 μm. E, Representative photomicrographs show superoxide production in the H89-treated mice (bottom left panel) and the vehicle-treated mice (bottom right panel) 1 hr after tFCI (n = 4 each). Superoxide production was observed by oxidized HEt signals (red), and nuclei were stained by DAPI (blue) 1 hr after reperfusion injury. The oxidized HEt signal was reduced in the SOD1 Tg mice compared with the wild-type mice (top panels). No conspicuous difference was observed between the H89-treated mice and the vehicle-treated mice (bottom panels). Scale bar, 25 μm.
