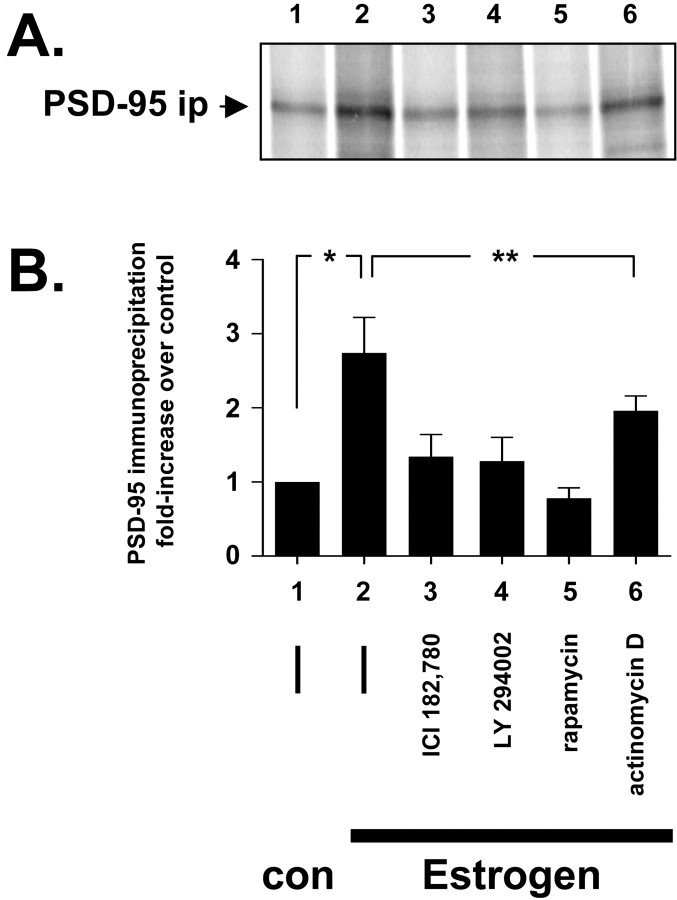
Fig. 4.
PSD-95 new protein synthesis. Metabolically [35S-Met/Cys] pulse-labeled dNG108-15 neurons were preincubated for 1 hr with either inhibitor (lane 3, 100 nm ICI 182,780; lane 4, 50 μmLY294002; lane 5, 10 nm rapamycin;lane 6, 4 μm actinomycin D) or (—) inhibitor diluent control (equivalent volume DMSO, lanes 1 and 2). Then, the neurons were either treated for an additional 6 hr with β-cyclodextrin control (con, lane 1) or 10 nm17β-estradiol (Estrogen, lanes 2–6). The cells were harvested in ice-cold RIPA buffer, and the cleared extract was immunoprecipitated for PSD-95 protein. After stringent washing, the immunoprecipitate was run by 7.5% SDS-PAGE, and the dried acrylamide gel was then exposed to a phosphorimage screen for densitometry. Only newly synthesized PSD-95 protein with [35S] incorporation is captured by the phosphorimaging screen. A, The PSD-95 immunoprecipitation (PSD-95 ip); B, the corresponding densitometry analysis. In the absence of inhibitors, estrogen stimulates an approximate threefold increase in new PSD-95 protein synthesis (significantly greater than control; Student's two-tailed t test; *p < 0.05). This protein synthesis is reduced to near control levels (lane 1) by either the ERα antagonist ICI 182,780 (lane 3) or the PI3K inhibitor LY294002 (lane 3). Rapamycin is a potent mTOR kinase inhibitor and can inhibit protein synthesis, and it reduces estrogen-stimulated PSD-95 protein synthesis to below control levels (lane 5). Actinomycin D inhibits mRNA transcription and decreases slightly estrogen-stimulated PSD-95 new protein synthesis (lane 6). However, this decrease is not statistically significant (Student's two-tailedt test; **p > 0.05) and suggests that PSD-95 protein synthesis is transcription independent.