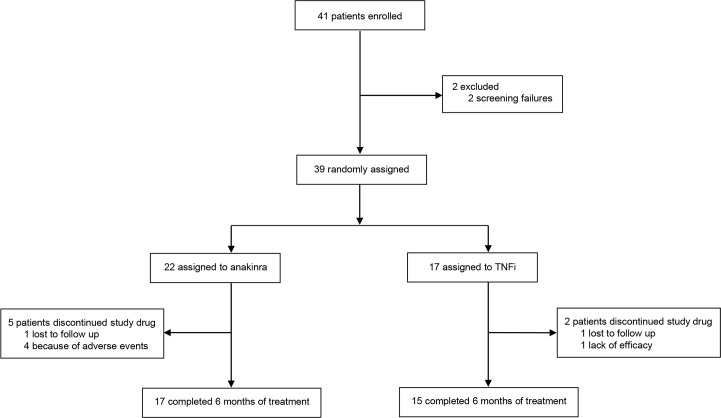
Fig 1. Trial profile.
Participants were recruited from June 2013 to March 2016 and were randomised to either once-daily recombinant human interleukin-1-receptor antagonist (100 mg of anakinra) by daily subcutaneous self-administration or TNFi administered according to relevant data sheets. TNFi, tumour necrosis factor inhibitor.