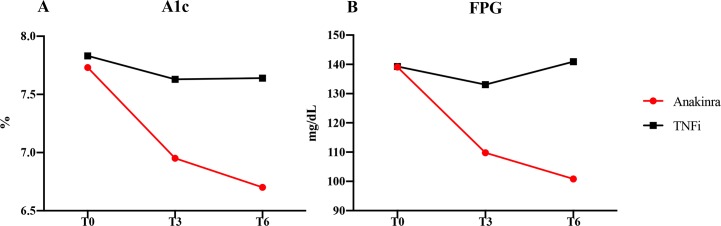
Fig 2. Measures of glycaemic control and bodyweight.
Metabolic measures of glycaemic control at baseline (T0) and after 3 (T3) and 6 (T6) months of treatment with anakinra or TNFi. (A) HbA1c%. T0: anakinra group (7.73% ± 0.67) versus TNFi group (7.83% ± 0.76); T3: anakinra group (6.95% ± 0.61) versus TNFi group (7.63% ± 0.68), p = 0.0038; T6: anakinra group (6.70% ± 0.67) versus TNFi group (7.64% ± 0.65), p < 0.001. (B) FPG. T0: anakinra group (139.05 ± 50.09 mg/dL) versus TNFi group (139.25 ± 29.55 mg/dL); T3: anakinra group (109.78 ± 30.58 mg/dL) versus TNFi group (133.06 ± 27.72 mg/dL), p = 0.027; T6: anakinra group (100.81 ± 11.11 mg/dL) versus TNFi group (140.93 ± 39.45 mg/dL), p < 0.001. FPG, fasting plasma glucose; HbA1c%, percentage of glycated haemoglobin; TNFi, tumour necrosis factor inhibitor.