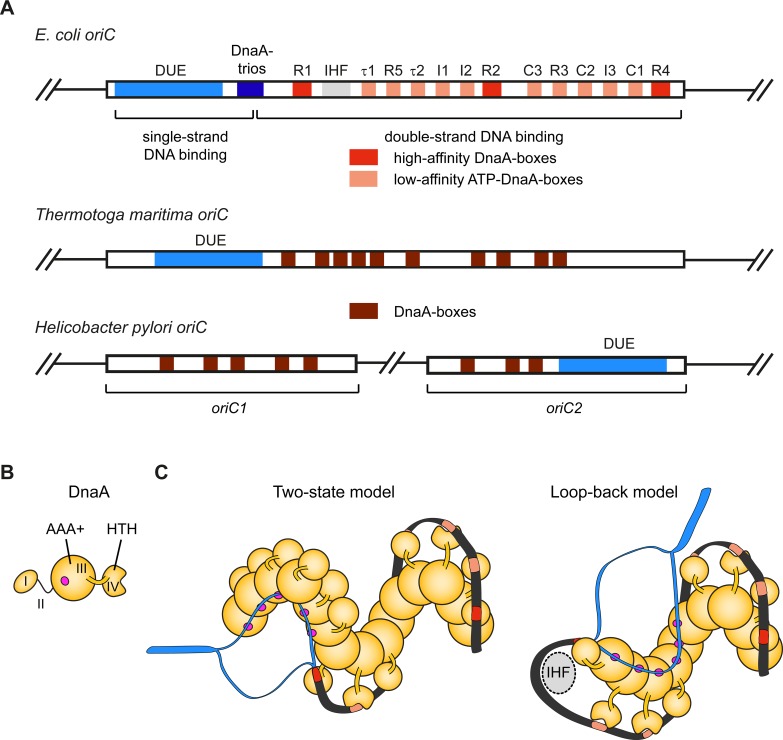
Fig 2. Origin organization and recognition in bacteria.
A) Schematic of the architecture of E. coli origin oriC, Thermotoga maritima oriC, and the bipartite origin in Helicobacter pylori. The DUE is flanked on one side by several high- and weak-affinity DnaA-boxes as indicated for E. coli oriC. B) Domain organization of the E. coli initiator DnaA. The magenta circle indicates the single-strand DNA binding site. C) Models for origin recognition and melting by DnaA. In the two-state model (left panel), the DnaA protomers transition from a dsDNA binding mode (mediated by the HTH-domains recognizing DnaA-boxes) to an ssDNA binding mode (mediated by the AAA+ domains). In the loop-back model, the DNA is sharply bent backwards onto the DnaA filament (facilitated by the regulatory protein IHF [40]) so that a single protomer binds both duplex and single-stranded regions. In either instance, the DnaA filament melts the DNA duplex and stabilizes the initiation bubble prior to loading of the replicative helicase (DnaB in E. coli). HTH–helix-turn-helix domain, DUE–DNA unwinding element, IHF–integration host factor.