Fig. 10.
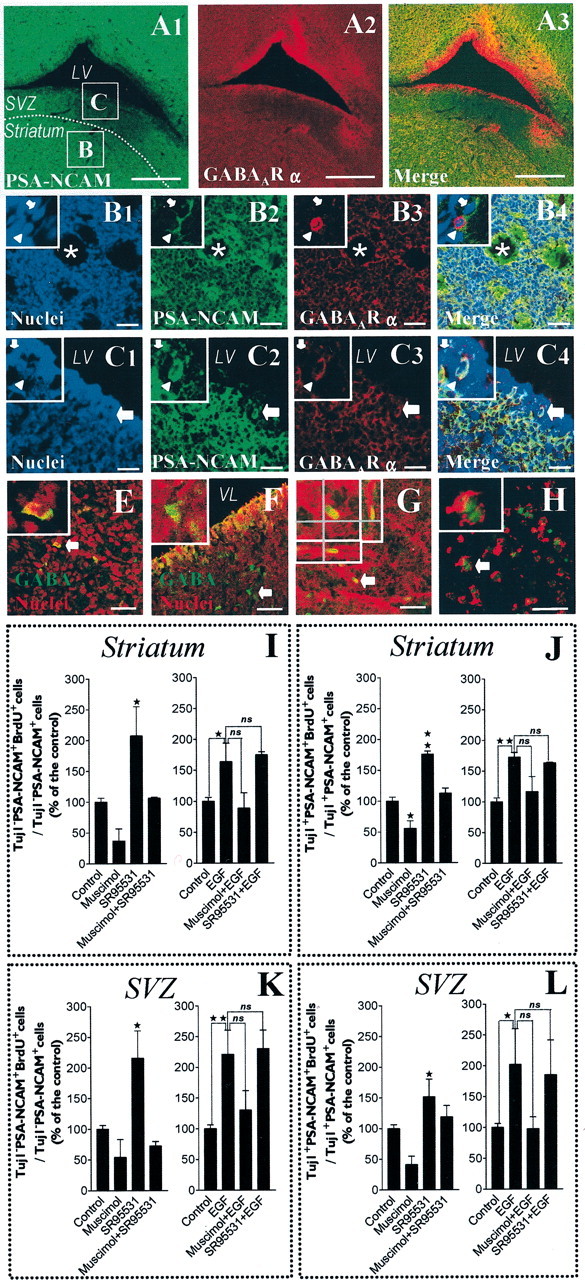
GABAAR expression and activation in brain slices: the activation of GABAAR inhibits the proliferation of PSA-NCAM+ cells in the postnatal striatum and adjacent SVZ. A1–3, Confocal single plane images of immunohistochemical stainings (30-μm-thick tissue sections) showing a field containing the striatum separated from the subventricular zone (SVZ) by a white dotted line and bordered by the lateral ventricle (LV). PSA-NCAM staining appears in green (A1), GABAAR α appears in red (A2), and merge of A1 andA2 appears in A3. B1–4, High-magnification views of the field delimited by the boxed areaB of A1, which is a representative field of the striatum, with nuclei in blue (B1), PSA-NCAM in green (B2), GABAAR α in red (B3), and merge of B1, B2, and B3 in B4. Insets display two PSA-NCAM+ cells (high magnification) that are immunoreactive (arrowhead) or not immunoreactive (arrow), respectively, for GABAAR α. C1–4, High-magnification views of the field delimited by the boxed area C ofA1, which is a representative field of the SVZ, with nuclei in blue (C1), PSA-NCAM in green (C2), GABAAR α in red (C3), and merge of C1, C2, andC3 in C4. Insets show two PSA-NCAM+ cells (high magnification) immunoreactive (arrowhead) or not immunoreactive (arrow), respectively, for GABAAR α. E, F, Confocal images showing immunostaining of a striatal area (E) and an SVZ area (F) with nuclei in red and GABA staining in green. Insets display a GABA+ cell (arrow in full image) at higher magnification. G, Proliferation assay in acutely dissected organotypic tissue slices from postnatal striatum (Z-series confocal image) treated with EGF. We show BrdU (green) immunostaining in a PSA-NCAM (red)-expressing cell of a striatal slice after 18 hr of BrdU incorporation. Inset displays one cell (corresponding to the arrow in the full image) viewed as stacked Z-dimension images, comprising 0.5 μm optical sections taken 3 μm apart. The Z-dimension reconstruction was also observed orthogonally in both X–Z and Y–Z planes that are shown under and to the right of each Z-dimension composite, respectively. H, Confocal image of acutely isolated cells derived from mechanical dissociation of the striatal part of 400-μm-thick tissue slices at the end of the BrdU incorporation assay. These cells were immunostained for PSA-NCAM (red) and BrdU (green). Scale bars: C1–4, 30 μm;B1–4, E–H, 40 μm;A1–3, 500 μm. I–L, Histograms showing BrdU labeling indexes in Tuj1−/PSA-NCAM+ (I, K) and Tuj1+/PSA-NCAM+ (J, L) cells from the striatum (I, J) and the SVZ (K, L), as defined in A1. Striatal and SVZ areas were separated by microdissection of organotypic slices, placed in the same well, and then incubated with drugs and BrdU (20 μm) for 18 hr. In EGF-free medium, 100 μmmuscimol inhibited the incorporation of BrdU in Tuj1−/PSA-NCAM+ and Tuj1+/PSA-NCAM+ cells from the striatum (I, J) and from the SVZ (K, L). These effects were totally abolished by SR-95531 (10 μm). Moreover, when applied alone, SR-95531 significantly increased the incorporation of BrdU in Tuj1−/PSA-NCAM+ cells and in Tuj1+/PSA-NCAM+ cells from the striatum (I, J) and from the SVZ (K, L) (n = 2–4; ANOVA-1 followed by a Dunnett's post-test; *p < 0.05,**p < 0.01). EGF (20 ng/ml) significantly increased the incorporation of BrdU in Tuj1−/PSA-NCAM+ and Tuj1+/PSA-NCAM+ cells from the striatum (I, J) and from the SVZ (K, L). In EGF-containing medium, muscimol exerted similar but less significant effects than in EGF-free conditions, and SR95531 had no effect when applied alone (n = 2–5; Student'st test; *p < 0.05, **p < 0.01).
