Fig 2.
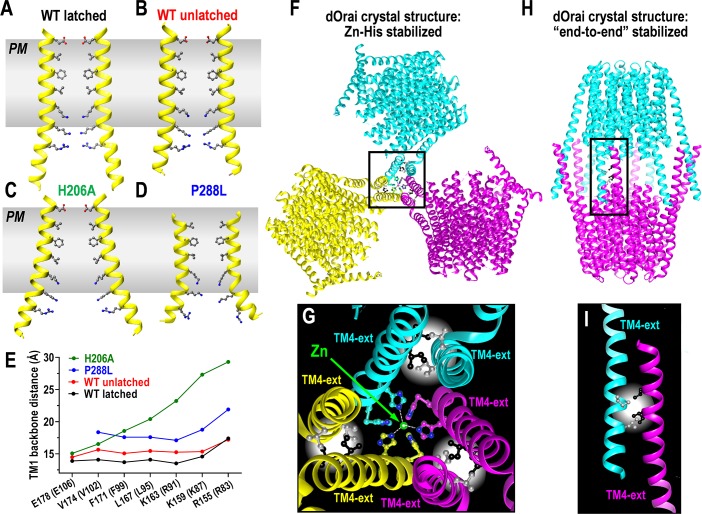
(A–D) Crystal-derived structures of the TM1 pore-forming helix in dOrai. Structures are shown for the WT dOrai channel derived from (A) the latched/paired dOrai WT closed-channel crystal structure [14]; (B) the unlatched/unpaired dOrai WT closed-channel crystal structure [11]; (C) the unlatched/unpaired dOrai-H206A open-channel crystal structure [11]; (D) the unlatched/unpaired dOrai-P288L open-channel crystal structure [11]. (E) Distances between the peptide backbones of the pore-lining residues across the opposite-facing TM1 helices for each of the pore structures shown in (A–D). (F) Crystal packing of the dOrai WT channel in a latched/paired configuration stabilized through Zn2+-mediated trimeric association of His-pairs at the C terminus of TM4e helices [14]. (G) Detailed structure of the Zn2+-mediated trimeric association of TM4e helices in (F) showing the Zn2+-binding to H330 and H334, and the antiparallel association of I316 and L319 residues in the crystal structure. Note the 3 His-pairs are from only 3 of the 6 TM4e helices, and their Zn2+-binding may contribute to the asymmetric pairing of TM4e helices observed in the crystal structure. (H) Crystal packing of the dOrai WT channel in a unlatched/unpaired configuration stabilized through “end-to-end” TM4e interactions [11], similar to the crystal packing identified for dOrai-P288L [12]. (I) Detailed structure of the association of TM4e helices in the “end-to-end” crystal packing in (H) showing the antiparallel association of I316 and L319 residues. dOrai, Drosophila Orai; PM, plasma membrane; TM, transmembrane; TM4e, TM4 extesion; WT, wild type.