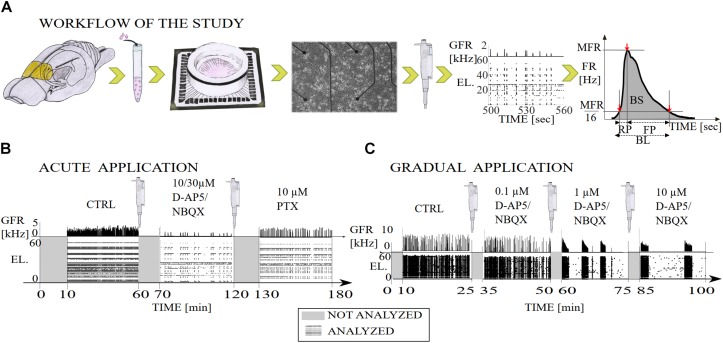
FIGURE 1.
Illustration of the workflow of the study and diagrams of the experimental recording protocols with pharmacological procedures. (A) The primary cell cultures were extracted from the prefrontal cortex of the postnatal brains of Wistar rats (yellow). The cortical cells were derived from the tissue by mechanical chopping, trypsinization and dissociation, the cells were seeded on polyethyleneimine coated MEA dishes and cultured until 3 weeks in vitro. Network-wide activity was recorded with MEA before and after either the gradual or acute application of excitatory or inhibitory synaptic receptor antagonists. Data analysis was performed on the multi-unit spike times, including NB detection, computation of the burst measures, such as BL, RP, FP, MFR, BS, and RC. Additionally, we computed interspike interval distributions (ISI), interburst-interval distributions (IBI) and cumulative time curves of electrode recruitment (see section “Materials and Methods”). (B) Timeline of a total of 180 min of recording in which excitatory synaptic transmission was probed by acute application of either AMPAR or NMDAR antagonist (NBQX and D-AP5, respectively both 10 μM), and inhibitory synaptic transmission was probed by acute application of GABAAR antagonist (PTX, 10 μM). After application of an antagonist the first ten minutes of the recording were not analyzed and the remaining 50 min of the recording were analyzed. (C) Timeline of a total of 100 min of recording in which excitatory synaptic transmission was probed by gradual application of AMPAR (NBQX, 0.1, 1, and 10 μM) or NMDAR (D-AP5 0.1, 1, and 10 μM) antagonist. The first ten minutes of the recording were not analyzed due to the transition phase (gray) and the remaining 15 min were analyzed (raster plot with global firing rate [kHz]). GFR denotes the global firing rate and EL the electrode numbers.