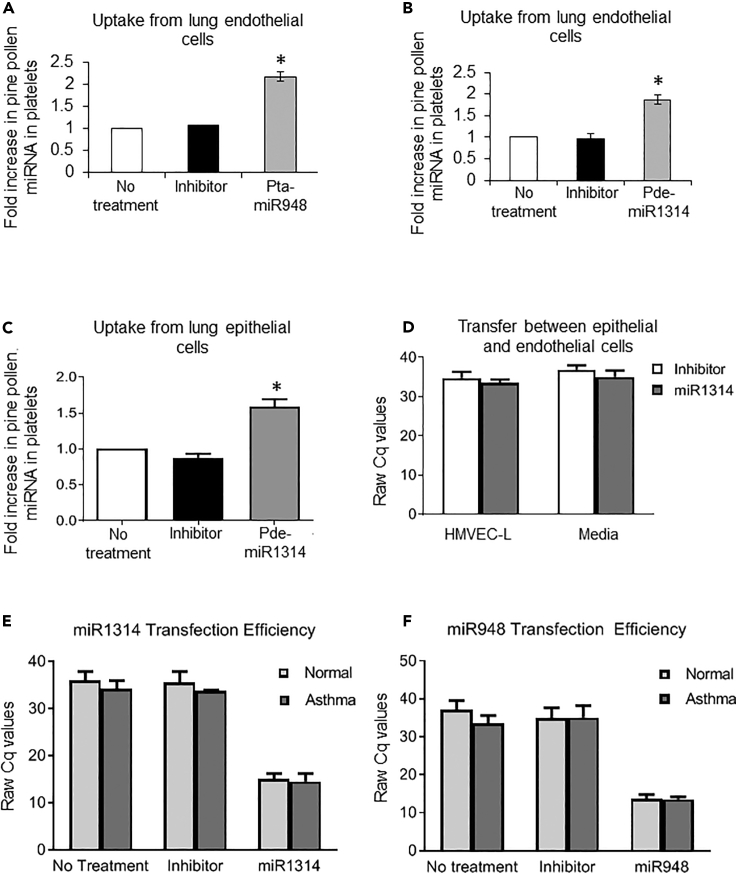
Figure 2.
Transfer of Pollen miRNA between Platelets and Relevant Pulmonary and Vascular Cells
(A–D) Endothelial or epithelial cells were transfected with the respective synthetic pollen miRNA, and platelets were circulated over the cells for 10 min. miRNA presence after incubation was analyzed by RT-qPCR. Uptake of (A) pta-miR948 (raw Cq values of no treatment 32.9 ± 7; inhibitor 31.9 ± 5; miR1314 16.5 ± 1) and (B) pde-miR1314 (raw Cq values of no treatment 30.5 ± 4; inhibitor 30.4 ± 5; miR1314 16.3 ± 2) by platelets from endothelial cells (HMVEC-L) and of (C) pde-miR1314 (raw Cq values of no treatment 31.6 ± 1; inhibitor 33.2 ± 2; miR1314 18.3 ± 1) from epithelial cells (NHBE). Platelets in (A) and (B) were isolated from three donors (F, age 23 years; M, age 49 years; F, age 22 years) and in (C) also from three donors (F age 35 years; F age 43 years; M age 42 years). (D) Transfer of pde-miR1314 from transfected epithelial cells to endothelial cells in co-culture transwell system was not significant. Each condition was normalized to the baseline pollen pde-miR1314 content in each person's platelets without treatment.
(E and F) Transfection efficiency for synthetic pollen miRNAs used in this study. Epithelial cells were transfected with pollen miRNA and tested for mRNA expression by qPCR. Transfection efficiency was established for (E) pde-miR1314 and (F) pta-miR948 pollen miRNAs using primary human cells derived from normal (NHBE) and asthmatic (D-HBE-As) sources. Similar results were observed for HMVEC-L cells.
Data in each graph are representative of means ±SEM for each condition; significance in (A), (B), and (C) was assessed by one-way ANOVA, followed by Bonferroni post-test; significance in (D), (E), and (F) was assesed by two-way ANOVA and star symbol (*) indicates p < 0.05.