Abstract
Increasing evidence indicates that paternal diet can result in metabolic changes in offspring, but the definite mechanism remains unclear in birds. Here, we fed breeder cocks five different diets containing 0, 0.25, 1.25, 2.50 and 5.00 mg kg−1 folate throughout life. Paternal folate supplementation (FS) was beneficial to the growth and organ development of broiler offspring. Most importantly, the lipid and glucose metabolism of breeder cocks and broiler offspring were affected by paternal FS, according to biochemical and metabolomic analyses. We further employed global analyses of hepatic and spermatozoal messenger RNA (mRNA), long non-coding RNA (lncRNA) and micro RNA (miRNA). Some key genes involved in the glycolysis or gluconeogenesis pathway and the PPAR signalling pathway, including PEPCK, ANGPTL4 and THRSP, were regulated by differentially expressed hepatic and spermatozoal miRNAs and lncRNAs in breeder cocks and broiler offspring. Moreover, the expression of ANGPTL4 could also be regulated by differentially expressed miRNAs and lncRNAs in spermatozoa via competitive endogenous RNA (ceRNA) mechanisms. Overall, this model suggests that paternal folate could transgenerationally regulate lipid and glucose metabolism in broiler offspring and the epigenetic transmission may involve altered spermatozoal miRNAs and lncRNAs.
Keywords: folate, miRNA, lncRNA, paternal transgenerational epigenetics, glycolysis or gluconeogenesis, PPAR signalling pathway
1. Background
Increasing evidence from Drosophila melanogaster, zebrafish and mammals suggests that epigenetic marks passed on by the sperm allow for the inheritance of information on paternal environmental exposure [1–3]. Mechanistically, altered DNA methylation, histone modifications and chromatin state have been reported to mediate transgenerational effects [2–4]. Recently, several studies have reported the transgenerational transmission of maternal experiences in chickens and other birds and implied that DNA methylation could mediate the maternal transgenerational effect [5,6]. However, detailed information on paternal transgenerational regulatory effects, especially the epigenetic marks that mediate paternal transgenerational effects, is still not yet illustrated in birds. Micro RNAs (miRNAs) regulate DNA methylation, histone modifications and messenger RNA (mRNA) expression and can induce non-Mendelian transgenerational inheritance in mammals [7]. Long non-coding RNAs (lncRNAs) could also regulate several epigenetic modifications, including miRNA and mRNA expression [8,9]. Moreover, changes in environmental exposure, especially altered nutritional supplementation, could also change lncRNA expression and further influence target gene expression [10]. Thus, the miRNAs or lncRNAs could serve as the underlying paternal transgenerational epigenetic mechanism in birds.
Folate could serve as an important carrier of methyl groups in one carbon metabolism pathway and further influence epigenetic processes, amino acid metabolism, nucleotide synthesis and lipid metabolism [11]. It has been well established that maternal folate supplementation (FS) during pregnancy could regulate the development and health of offspring [12]. Additionally, a recent study identified that paternal folate deficiency is associated with increased birth defects, and DNA methylation could serve as the epigenetic marker passed on by the sperm [13]. However, the underlying mechanism through which epigenetic modification acts in the intergenerational inheritance of paternal folate metabolism needs to be further studied.
Hence, we hypothesized that miRNAs and lncRNAs could show altered expression profiles in response to different paternal diets and transmit certain metabolic changes from father to offspring. By using a chicken model that was fed different concentrations of folate throughout life, we addressed how paternal FS exerts a transgenerational effect on birds, and we further studied the underlying mechanisms.
2. Material and methods
(a). Animals and experimental design
A total of 200 1-day-old Arbor Acres breeder cocks (Beijing Arbor Acres Poultry Breeding Co. Ltd. China) were randomly assigned to five treatments with five replicates per treatment and eight birds per replicate. The breeder cocks from each treatment were fed a standard diet mainly consisting of corn and soya bean meal (electronic supplementary material, table S1). The only difference among treatments was the concentration of folate (Sigma, USA) in the feed (0, 0.25, 1.25, 2.50 and 5.00 mg folate kg−1 feed, named the CON, FS1, FS2, FS3 and FS4 groups, respectively). Additionally, 600 1-day-old Arbor Acres breeder hens were synchronously fed corn–soya bean meals containing 1.2 mg kg−1 folate. At 32 weeks of age, one cock from each replicate was selected and mated with 16 hens by artificial insemination. At 35 weeks of age, the fertile eggs of five consecutive days from each replication of each treatment were collected and then artificially hatched. The newborn broilers were grouped according to their paternal diets (eight randomly selected offspring birds from the same cocks were fed as a replicate) and raised to 21 days by feeding corn–soya bean meal (electronic supplementary material, table S2) without extra folate. The water, diet and photoperiod were set according to the recommendations established by Aviagen Broiler Breeders Company, USA. The body weight (BW) was recorded. The fertilization rate and hatchability of fertile eggs were recorded. At 35 weeks of age, four breeder cocks from each replicate were randomly selected. Semen samples were collected, and then the volume of ejaculation and the spermatozoa quality were immediately measured according to Hu et al. [14].
(b). Sample collection
From 32 to 40 weeks of age, the semen from the selected cocks was gathered and immediately frozen in liquid nitrogen every two weeks, and five semen samples from the same bird were equally mixed together for further analyses.
In order to collect enough blood sample from the newborn chicks for further analysis, four 1-day-old offspring chicks from the same replicate were selected for sample collection. Herein, the blood and tissue samples of 40-week-old cocks (one bird per replicate), 1-day-old offspring chicks (four birds per replicate) and 21-day-old broiler offspring (one bird per replicate) were gathered. The bird from each replicate was randomly selected and weighed after fasting for 12 h. Blood samples were collected from the brachial vein and withdrawn into heparinized tubes. Plasma samples were separated by centrifugation at 3500g for 15 min at 4°C, and the supernatant was dispensed into 1.5 ml centrifuge tubes. Specifically, the plasma sample of four 1-day-old offspring chicks from same replicate were equally and equably mixed into one. Then, these birds were euthanized by exsanguination after the intravenous administration of 3% sodium pentobarbital (25 mg kg−1 BW; Sigma, USA) and immediately dissected. The organs (liver, thymus, spleen and bursa) were collected and immediately weighed. Organ indexes were expressed relative to BW (g kg−1). The left lobe of the livers at the same position without blood contamination were collected and immediately frozen in liquid nitrogen. The length of the duodenal, jejunal, ileal and caecal segments were recorded and expressed relative to BW. Furthermore, by removing the contaminating intestinal contents, the middle complete duodenal, jejunal and ileal segments with a length of 3 cm were collected and fixed in 10% buffered formalin for at least 48 h for further histology processing.
(c). Intestinal morphology
After fixation, the fixed samples were dehydrated and cleared. Then, intestinal samples were cut and inserted into cassettes that were embedded in liquid paraffin. Then, a 5 µm paraffin section was obtained using a microtome and stained with haematoxylin-eosin. Villus height and crypt depth were then determined [15]. Briefly, the height or depth of three different villi or crypts of each microscopic field and 10 microscopic fields for each fixed sample were measured.
(d). Biochemical assay of liver and plasma samples
The concentration of total protein, malondialdehyde, total glyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and free fatty acid (FFA), the total antioxidant capacity (T-AOC), and the activities of phosphoenolpyruvate carboxykinase (PEPCK), 6-phosphofructokinase (PFK), pyruvate kinase (PK), hexokinase (HK) and citroyl synthetase (CS), were measured with spectrophotometric methods using commercial kits (Jiancheng Biological Engineering Research Institute, China). The concentrations of leptin, adiponectin, carnitine, malate dehydrogenase (MDH) and hepatic lipase (HL) were measured with ELISA commercial kits (Cloud-Clone Corporation, Houston, USA).
(e). Gas chromatography-mass spectrometry metabolomic quantification of liver samples
Approximately 50 mg liver samples of breeder cocks and 1-day-old and 21-day-old broiler offspring from the CON and FS2 treatments were preprocessed for metabolomic analyses. The derivatives of the sample were analysed and the acquired gas chromatography-mass spectrometry data were processed as described in a previous study [16].
(f). RNA isolation
Total RNA from the sperm samples of breeder cocks and the liver samples of breeder cocks and 1-day-old broilers from the CON and FS2 groups were separately extracted using Phenol Water (Solarbio, Beijing, China) and TRIzol reagent (Invitrogen, CA, USA).
(g). miRNAs, lncRNAs and mRNAs sequencing and integrated analysis
Approximately 3 µg of total RNA was used to prepare an lncRNA and mRNA library for paired-end sequencing (2 × 150 bp) on an Illumina Hiseq2500 in accordance with a previous study [17]. The screening of differentially expressed mRNAs (DEmRs) and lncRNAs (DElRs) (with criteria of p-value < 0.05 and log2foldchange >1 or <−1) as well as the prediction of target genes of DElRs were performed in accordance with the previous description [18]. Then, approximately 1 µg of total RNA from each prepared RNA sample was used to prepare small RNA libraries and perform single-end sequencing (1 × 50 bp). The identification of differentially expressed miRNAs (DEmiRs) (with criteria of p-value <0.05 and log2foldchange >1 or <−1) as well as the prediction of target genes of DEmiRs were performed according to previously described methods [7,19]. Functional annotation was performed based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database by using KOBAS software [20], and the significance threshold was set as p < 0.05. Furthermore, the competitive endogenous RNA (ceRNA) mechanism among hepatic DEmRs of offspring and spermatozoal DEmiRs and DElRs of breeder cocks were identified [21]. Specifically, the details for all different bioinformatic analysis and the detailed parameters used in each step for miRNA, mRNA and lncRNA sequencing analyses are all shown in the electronic supplementary material, figure S1.
(h). 3′untranslated region luciferase reporter assays
The 3′untranslated region (UTR) of PEPCK or ANGPTL4 mRNA containing the binding site of PC-5p-30232_97 or PC-3p-448507_5, respectively, was amplified from the breeder cock livers' complementary DNA (cDNA) by polymerase chain reaction (PCR) with the primers shown in the electronic supplementary material, table S3. The fragments were cloned into psiCHECKTM-2 vectors (Promega, Madison, WI, USA) at the 3′ end of the Renilla gene using XhoI and NotI restriction sites. After co-transfection of 500 ng of vector constructs with either 50 nM of PC-5p-30232_97 and PC-3p-448507_5 mimic or miR-NTC (RIBO Bio, Guangzhou, China) using 2.0 µl X-tremeGENE siRNA Transfection Reagent (Roche, USA) into HEK-293 T cells, the expression levels of PC-5p-30232_97 and PC-3p-448507_5 in cells were measured by quantitative real time-PCR (qRT-PCR) and then luciferase reporter experiments were performed [19].
(i). Culture of primary chicken hepatocytes and transfection of miRNA mimics
Hepatocytes were isolated from newly hatched male chickens and then cultured according to a previous description [22]. Either 50 nM of PC-5p-30232_97 and PC-3p-448507_5 mimic or miR-NTC (RIBO Bio, Guangzhou, China) was transfected into primary chicken hepatocytes, and the cells in the control group were left untreated. The expression levels of PC-5p-30232_97 or PC-3p-448507_5 and their targets (PEPCK or ANGPTL4) in the cells from the control, miR-NTC and miR-mimic (PC-5p-30232_97 or PC-3p-448507_5) groups were measured by qRT-PCR.
(j). Real-time quantitative polymerase chain reaction
The miRNA was reverse transcribed using a Bulge-Loop™ miRNA miR-Reverse Primer Set (RIBO Bio, China). The mRNA and lncRNA were reverse transcribed using a Prime Script® RT reagent Kit (Takara, China). Quantification was performed with iCycler IQ™ 5 (Bio-Rad, USA) using SYBR® Premix Ex Taq™ II (Takara, China). U6 and β-actin were separately used as internal normalization controls. The specific primers for the qRT-PCR of miRNAs were provided by the Bulge-Loop™ miRNA qRT-PCR Primer Set. The specific primers for the qRT-PCR of lncRNAs and mRNAs are listed in the electronic supplementary material, table S3. All samples were examined in triplicate. All data were analysed using the 2−ΔΔCt method [23].
(k). Statistical analysis
The statistical evaluation was performed by Student's t or ANOVA test using SPSS 21.0. If a significant treatment effect was observed by the ANOVA, the significance of the differences between treatments was identified by Duncan's multiple comparisons test. All data are expressed as the mean and standard error. Differences were considered to be statistically significant at p < 0.05.
3. Results and discussion
(a). Phenotypic changes in breeder cocks and their broiler offspring resulted from the dietary folate supplementation of breeder cocks
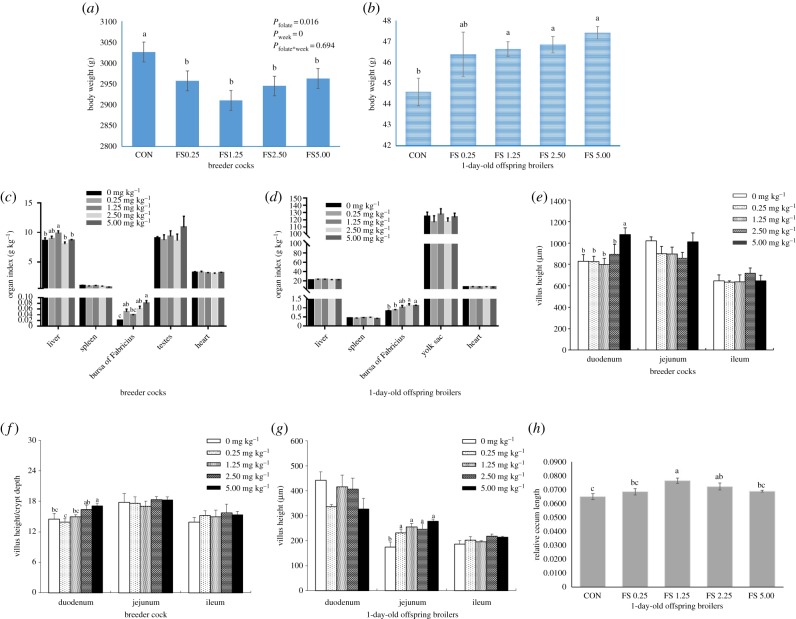
Breeder cocks with extra folate exhibited a reduced BW (figure 1a) and increased organ indexes of the liver and bursa of Fabricius (figure 1c). Additionally, duodenum morphology, namely, the villus height and the radio of the villus height to crypt depth (V/C), was significantly increased in the FS groups (figure 1e,f). Regarding the broiler offspring, the birth weight of the 1-day-old broilers in the paternal folate supplementation (PFS) groups was significantly increased (figure 1b). Moreover, the organ index of the bursa of Fabricius of 1-day-old and 21-day-old broiler offspring was significantly increased in PFS groups, but the indexes of the livers and hearts of 21-day-old broiler offspring were significantly decreased (figure 1d). Furthermore, we also found that the intestinal development of the broiler offspring in the PFS groups, including the villus height of the jejunum and the relative caecum length of 1-day-old broiler offspring, were all significantly increased (figure 1g,h).
Figure 1.
Transgenerational developmental changes in breeder cocks and their broiler offspring induced by dietary FS in breeder cocks. (a,b) Effects on BW. (c,d) Effects on organ indexes. (e–g) Effects on intestinal morphology. (h) Effects on relative caecum length. Superscripts (a and b) denote significant differences in the same index. (Online version in colour.)
Considering that these phenotypic changes might result from the effect of folate on sperm quality [24], the fertilization rate of the hatching eggs and all the sperm characteristic of the breeder cocks were measured, but were found to not be significantly affected (electronic supplementary material, table S4). However, the hatchability of fertile eggs was significantly increased in the PFS groups (electronic supplementary material, table S4). Overall, the roles of folate in transgenerationally regulating the development of the fetus was also identified, which again proved that PFS was crucial to embryonic development [12,13].
(b). Dietary folate supplementation causes the transgenerational inheritance of changes in lipid and glucose metabolism
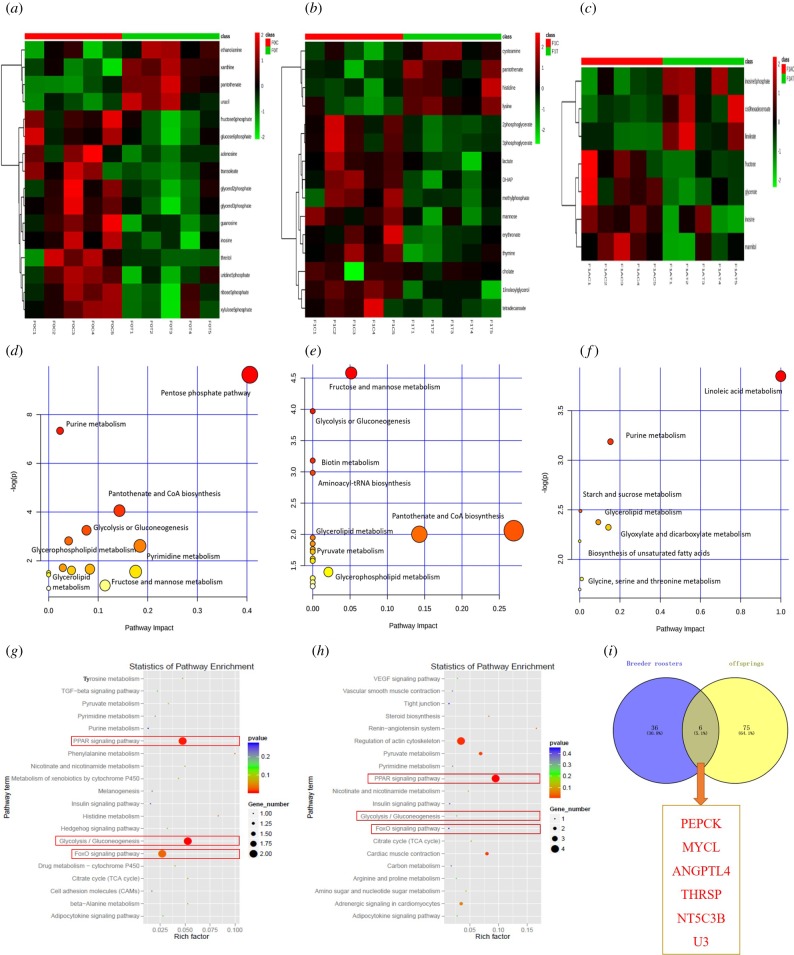
The differences in the hepatic metabolome of the chickens were separately compared and a dramatic difference between the livers of the CON and FS2 groups was identified (electronic supplementary material, figure S2). A total of 16, 15 and seven significantly differential hepatic metabolites were separately identified, and most altered metabolites were involved in lipid and glucose metabolic processes (figure 2a–c). Several altered pathways, including the pentose phosphate pathway, purine metabolism, pantothenate and CoA biosynthesis, and the glycolysis or gluconeogenesis pathways in breeder cocks (figure 2d), fructose and mannose metabolism, glycolysis or gluconeogenesis, and biotin metabolism pathways in 1-day-old broiler offspring (figure 2e), and linoleic acid metabolism, purine metabolism, starch and sucrose metabolism, and glycerolipid metabolism pathways in 21-day-old broiler offspring (figure 2f), were identified across generations. Taken together, the data suggest that PFS could mainly regulate or transgenerationally affect lipid metabolism and glycometabolism in breeder cock and broiler offspring.
Figure 2.
Altered hepatic metabolomic profiles and transcriptomics profiles of breeder cocks and broiler offspring induced by dietary FS in breeder cocks. (a–c) Altered hepatic metabolomic profiles of breeder cocks and 1-day-old and 21-day-old broiler offspring; the upregulated metabolites are depicted in red, whereas the downregulated metabolites are depicted in green. (d–f) Significantly enriched metabolic pathways based on the differential metabolites. (g,h) Significantly enriched pathways in breeder cocks and 1-day-old broiler offspring based on hepatic differentially expressed genes. (i) Hepatic co-differentially expressed genes between breeder cocks and broiler offspring. (Online version in colour.)
In plasma, significant decreases in HDL-C, LDL-C, VLDL-C and adiponectin were identified in breeder cocks and broiler offspring in the FS groups compared with those in the control group. Moreover, significantly increased TG and FFA were also identified in the 1-day-old broiler offspring of the PFS groups (electronic supplementary material, table S5). In livers, significantly increased TC and FFA were identified in the breeder cocks and broiler offspring in the FS groups compared with those in the control group. Additionally, significant increases in TG, HDL-C and carnitine in breeder cocks and a significant increase in MDH in 1-day-old broiler offspring were observed in the FS groups (electronic supplementary material, table S5). Regarding hepatic glycometabolism, the activities of PEPCK in breeder cocks and 1-day-old broiler offspring, PK in 1-day-old and 21-day-old broiler offspring, and CS in breeder cocks and 21-day-old broiler offspring were all significantly increased in the FS groups (electronic supplementary material, table S5). In parallel, antioxidant capacity could be influenced by the pentose phosphate pathway and lipid metabolism [25]. The T-AOC of breeder cocks and 1-day-old and 21-day-old broiler offspring were all increased in the FS groups. Moreover, the concentration of malondialdehyde was not changed (electronic supplementary material, figure S3), which indicated that the antioxidant ability of breeder cocks and their offspring in FS groups were significantly enhanced.
The effect of dietary FS on lipid metabolism is widely disputed [11]. Previous studies have mostly shown that folate could promote lipoclastic activity by increasing the biosynthesis of phosphatidylcholine and carnitine and by decreasing the expression of lipid synthesis-related genes [11]. However, several recent studies have found that chronic FS could induce increased inactive folate in the body and further induce lipopexia in the liver [26,27]. The present study found that chronic FS could actually influence the lipid metabolic process by influencing glycerophospholipid metabolism, glycerolipid metabolism and fatty acid biosynthesis. However, the hepatic TG contents were increased in the breeder cocks and their offspring from the PFS groups, which was in accordance with previous studies [26,27]. Additionally, enhanced glucose metabolic functions, especially glycolysis and gluconeogenesis functions, were identified in breeder cocks and their offspring from the FS groups. Hence, chronic FS could improve energy use by increasing gluconeogenesis and glycolysis and by reducing lipid catabolism.
(c). Folate supplementation induces transgenerational changes in the hepatic transcriptome
A total of 42 and 82 DEmRs induced by FS were identified in breeder cocks and 1-day-old broiler offspring (electronic supplementary material, figure S4A and S4B). Moreover, the qRT-PCR analyses proved that the transcriptomic analyses were reproducible and reliable (electronic supplementary material, figure S4C and S4D). The KEGG pathway analyses highlighted that the main changed pathways in breeder cocks and broiler offspring were related to lipid and glucose metabolism, including the PPAR signalling pathway, the FoxO signalling pathway, glycolysis/gluconeogenesis, pyruvate metabolism and the pentose phosphate pathway (figure 2g,h), which were consistent with the previous biochemical indexes and metabolomic analyses. Furthermore, the key genes involved in the effect of folate on lipid and glucose metabolism were also identified, including ALDH6, THRSP, PEPCK and ANGPTL4 in breeder cocks and THRSP, UbI, ME3, FABP5, PEPCK and ANGPTL4 in broiler offspring. Specifically, the PEPCK, ANGPTL4 and THRSP genes were co-DEmRs in breeder cock and broiler offspring (figure 2i).
The increased expression level or enzymatic activity of PEPCK was shown to enhance hepatic gluconeogenesis, which is consistent with our observations of enhanced gluconeogenesis in breeder cocks and broiler offspring from the FS groups [28]. The THRSP gene could participate in fatty acid synthesis and further influence fat deposition [29]. Similarly, ANGPTL4 was also identified as an adipokine involved in the regulation of lipid metabolism by inhibiting lipoprotein lipase activity and stimulating the lipolysis of white adipose tissue, resulting in increased levels of plasma triglycerides and fatty acids [30]. Furthermore, the PPAR signalling pathway [31] and the FoxO signalling pathway [32] are important pathways involved in the regulation of hepatic insulin sensitivity and lipid metabolism. Specifically, a previous study proved that the activation of the PPAR pathway could increase lipid storage and further enhance gluconeogenesis [31], which was in accordance with the increased hepatic TG concentration and increased hepatic PEPCK and ATGPTL4 expression in the present study. Overall, these results again indicated that paternal chronic dietary FS leads to the transgenerational inheritance of acquired glucose and lipid metabolic changes in broilers owing to the altered hepatic expression of PEPCK and ANGPTL4 and changes in the related pathways, including the PPAR signalling pathway, the FoxO signalling pathway, glycolysis/gluconeogenesis, pyruvate metabolism and the pentose phosphate pathway.
(d). Differential miRNA expression profiles contribute to the intergenerational inheritance of the effects of dietary folate supplementation on metabolism
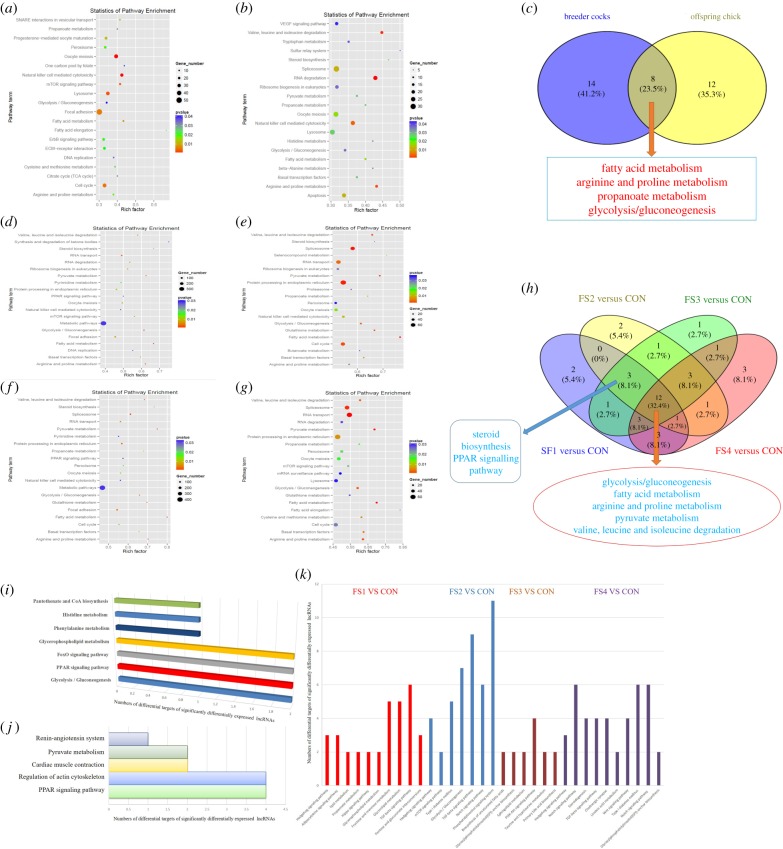
In livers, 31 and 23 DEmiRs between the FS2 and control groups were identified in breeder cocks and broiler offspring, respectively (electronic supplementary material, figure S5A and S5B). Based on their targets, several significantly enriched pathways involved in the regulation of lipid and glucose metabolism were also detected, including fatty acid metabolism, fatty acid elongation, TCA cycle and glycolysis/gluconeogenesis in breeder cocks (figure 3a) and fatty acid metabolism, steroid biosynthesis, propanoate metabolism, pyruvate metabolism and glycolysis/gluconeogenesis in broiler offspring (figure 3b). Of these significantly enriched pathways, fatty acid metabolism and glycolysis/gluconeogenesis were detected as significantly enriched pathways in both breeder cock and broiler offspring (figure 3c).
Figure 3.
Transgenerational regulatory roles of miRNAs and lncRNAs in regulating lipid and glucose metabolism in response to paternal FS. (a,b) Significantly enriched pathways based on targets of hepatic differentially expressed miRNAs (DEmiRs) in breeder cocks and broiler offspring. (c) Co-enriched pathways based on targets of hepatic DEmiRs between breeder cocks and broiler offspring. (d–g) Significantly enriched pathways based on targets of spermatozoal DEmiRs between the CON and other four FS groups. (h) Co-enriched pathways based on targets of spermatozoal DEmiRs among four different compared groups between the CON and other four FS groups. (i,j) Significantly enriched pathways based on targets of hepatic DElRs in breeder cocks and 1-day-old broiler offspring. (k) Significantly enriched pathways based on targets of spermatozoal DElRs between the CON and other four FS groups of breeder cocks. (Online version in colour.)
miRNAs have been shown to regulate gene expression after fertilization and may impact offspring development by acting on oocyte stores of maternal mRNA [33]. In sperm, 81, 115, 208 and 92 DEmiRs and 4294, 4715, 5256 and 4389 targets were detected in the four FS groups compared with the control group (electronic supplementary material, figure S5CS5F). Furthermore, KEGG analysis based on the DEmiRs of these four compared groups was performed and proved that the DEmiRs of the four compared groups were all be significantly enriched in several pathways related to lipid and glucose metabolism (figure 3d–g). Specifically, the glycolysis/gluconeogenesis, fatty acid metabolism, pyruvate metabolism and PPAR signalling pathways were the co-enriched pathways (figure 3h). Hence, we first confirmed that hepatic and spermatozoal DEmiRs could contribute to the regulation of DEmRs and related pathways of the fatty acid metabolism and glycolysis/gluconeogenesis, which were confirmed to be significantly enriched pathways based on hepatic DEmRs. Furthermore, qRT-PCR analyses of miRNAs in liver and sperm samples were performed and proved that our miRNA sequencing analyses were reproducible and reliable (electronic supplementary material, figure S5G). Overall, miRNAs are poised to be products of dynamic paternal FS and to uniquely contribute to post-fertilization gene expression, which is in accordance with a previous conclusion regarding paternal stress and trauma determined by using miRNA sequencing [33,34].
(e). Differentially expressed lncRNAs contribute to the potential transgenerational role of folate in offspring glucose and lipid metabolism
There were 64 and 21 hepatic DElRs detected in breeder cocks and broiler offspring, respectively (electronic supplementary material, figure S4E and S4F). Of these 64 DElRs in breeder cock livers, 29 DElRs could cis-regulate 46 DEmRs, and 64 DElRs could trans-regulate 42 DEmRs. Combining the trans-regulated targets with the cis-regulated targets, further KEGG analyses found that the identified lncRNAs could significantly regulate glycolysis/gluconeogenesis, the PPAR signalling pathway, the FoxO signalling pathway, phenylalanine metabolism and histidine metabolism (figure 3i). In 1-day-old broiler offspring, 21 DElRs induced by paternal dietary FS were also identified. Among these DElRs, 36 DEmRs could be cis-regulated by 15 DElRs, and 81 DEmRs could be trans-regulated by 21 DElRs. Our KEGG analyses also identified that these hepatic DElRs could regulate the target DEmRs in glycolysis/gluconeogenesis, the PPAR signalling pathway, the FoxO signalling pathway and pyruvate metabolism (figure 3j); these pathways were also confirmed to be significantly enriched pathways based on hepatic DEmRs. Hence, the DElRs contribute to the potential role of folate in glucose and lipid metabolism, which have been previously proven [35].
Devanapally et al. [36] reported that the non-coding RNAs (ncRNAs) of somatic cells could be transferred to sperm cells and further influence the target gene expression of the somatic cells of offspring during fertilization. Hence, the roles of DElRs in sperm were also confirmed. A total of 462, 633, 527 and 558 DElRs were identified in the four FS groups. Based on the 111, 261, 68 and 208 DEmRs identified in the four FS groups, we further predicted the roles of DElRs by combining the trans-regulated targets with the cis-regulated targets and performing KEGG analyses. The identified DElRs regulated the target mRNAs, which were mainly involved in glucose and lipid metabolism (figure 3k). These results revealed that spermatozoal lncRNAs could take part in the transgenerational inheritance of acquired glucose and lipid metabolic changes in broiler offspring in response to paternal FS.
(f). Integrated omics analysis
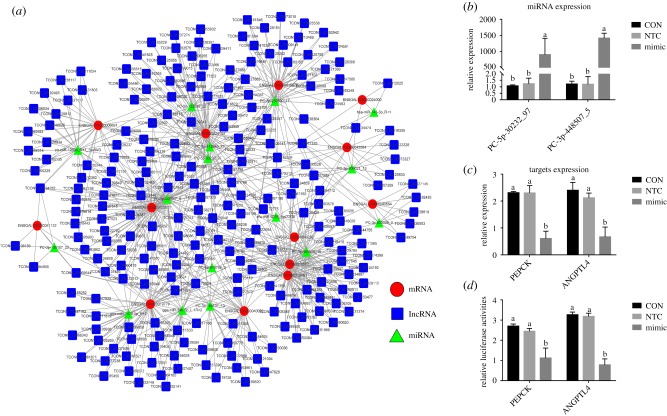
The PEPCK, ANGPTL4 and THRSP were co-differentially expressed in breeder cock and broiler offspring. Additionally, UbI, ME3 and FABP5, key genes in glycolysis/gluconeogenesis and the PPAR signalling pathway, were DEmRs in the broiler offspring. These six genes expressions could be regulated by the key DEmiRs and DElRs in breeder cock sperm (electronic supplementary material, table S6), which proved that the spermatozoal DEmiRs and DElRs induced by FS in breeder cocks could take part in the regulation of offspring gene expression. Another possibility is that mechanisms other than the direct effect of miRNAs and lncRNAs on target mRNA are involved, such as a ceRNA mechanism [37]. The differentially expressed mRNAs in the livers of offspring broilers, including RET, ANKRD1, TSPAN8, SPON1, CLIC3, SLC16A7, ANGPTL4, LIMK1, LAMA4, G0S2, ENPEP, TBC1D32 and SOAT1, could be regulated by the spermatozoal DEmiRs and DElRs through a ceRNA mechanism (figure 4a). Of these differentially expressed mRNAs, the ANGPTL4 (ENSGALT00000000877) could be co-regulated by PC-3p-448507_5 and 51 differentially expressed spermatozoal lncRNAs through ceRNA mechanisms (electronic supplementary material, table S7).
Figure 4.
Integrated omics analysis based on the identified differentially expressed spermatozoal miRNA and lncRNAs of breeder cocks and the hepatic mRNAs of their offspring broilers. (a) The ceRNA network among differentially expressed hepatic mRNAs of offspring broilers and the spermatozoal miRNA and lncRNAs of breeder cocks. (b) The expression of PC-5p-30232_97 and PC-3p-448507_5 in primary chicken hepatocytes after the transfection of these miRNA mimics and mimic-NTC. (c) The expression of PEPCK and ANGPTL4 in primary chicken hepatocytes after the transfection of PC-5p-30232_97 and PC-3p-448507_5. (d) Luciferase activities in luciferase reporter assays when PEPCK or ANGPTL4 3′UTR was co-transfected in 293T cells with a PC-5p-30232_97 or PC-3p-448507_5 mimic, respectively. (Online version in colour.)
The roles of two key miRNAs, PC-5p-30232_97 and PC-3p-448507_5, in regulating the expression of predicted target genes (PEPCK and ANGPTL4) were further tested. The transfection of these two miRNA mimics significantly increased their expression (figure 4b) and then separately decreased PEPCK and ANGPTL4 expression in primary chicken hepatocytes (figure 4c). Moreover, luciferase reporter assays showed a significant decrease in luciferase activity (p < 0.05) when the PEPCK or ANGPTL4 3′UTR was co-transfected in 293T cells with a PC-5p-30232_97 or PC-3p-448507_5 mimic, respectively (figure 4d). Overall, we lacked suitable methods to directly detect the effect of differentially expressed miRNAs and lncRNAs on the metabolic changes in offspring broilers, such as the microinjection of miRNAs or lncRNAs in the zygotes of mice [7,33]; we only studied the effect of differentially expressed miRNAs and lncRNAs by bioinformatics analysis and in vitro study [9], supporting the idea that epigenetic marks in spermatogenesis are dynamic and flexible and that they can thus be modified by nutrition. Therefore, the direct detection of the effect of differentially expressed miRNAs and lncRNA on the metabolic changes in offspring broilers, such as the microinjection of miRNAs or lncRNAs into the zygotes of mice, could be further performed in future studies.
4. Conclusion
The transgenerational effects observed in this study provide, to our knowledge, the first evidence that birds can modify their miRNA and lncRNA expression in response to different FS and transfer epigenetic changes through the male germline. These epigenetic changes are associated with altered transcriptomic and metabolomic profiles that lead to heritable alterations in metabolism, which may subsequently potentially affect the metabolism and growth of future generations. Thus, the results of this study suggested that different nutritional supplementation might induce long-lasting metabolic and developmental changes, which indicated that it is crucial to consider the transgenerational effect of nutritional supplementation when we study the suitable nutritional requirements of breeder cocks. Furthermore, the result of this study will also inform future transgenerational epigenetic research related to the roles of miRNAs and lncRNAs in mediating paternal transgenerational effects in birds.
Supplementary Material
Ethics
The use of animals and all experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Northwest A&F University (Yangling, Shaanxi, China).
Data accessibility
The sequence data have been deposited in Gene Expression Omnibus and can be accessed with the number GSE122759.
Competing interests
The authors declare no competing financial interests.
Funding
The work was supported by the National key research and development projects (grant nos. 2017YFD0500500 and 2017YFD0502200), the Program for Shaanxi science & technology (grant nos. 2017TSCXL-NY-04-04, 2018ZDCXL-NY-0201 and 2018ZDXM-NY-051-19), the China Postdoctoral Science Foundation (grant no. 2019M653774), the National natural science foundation of China (grant nos. 31001017 and 31272464), and the program for Yangling agricultural high-tech industries demonstration zone (grant no. 2018CXY-10).
References
- 1.Kishimoto S, Uno M, Okabe E, Nono M, Nishida E. 2017. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 8, 14031 ( 10.1038/ncomms14031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang L, et al. 2013. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell 153, 773–784. ( 10.1016/j.cell.2013.04.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Öst A, et al. 2014. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159, 1352–1364. ( 10.1016/j.cell.2014.11.005) [DOI] [PubMed] [Google Scholar]
- 4.Siklenka K, et al. 2015. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350, aab2006 ( 10.1126/science.aab2006) [DOI] [PubMed] [Google Scholar]
- 5.Leroux S, et al. 2017. Embryonic environment and transgenerational effects in quail. Genet. Sel. Evol. 49, 14 ( 10.1186/s12711-017-0292-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Yang N, Xu G, Liu S, Wang D, Song J, Duan Z, Yang S, Yu Y. 2018. Transgenerational transmission of maternal stimulatory experience in domesticated birds. FASEB J. 32, 7002–7017. ( 10.1096/fj.201800762RR) [DOI] [PubMed] [Google Scholar]
- 7.Wu L, et al. 2016. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell Metab. 23, 735–743. ( 10.1016/j.cmet.2016.01.014) [DOI] [PubMed] [Google Scholar]
- 8.Bao X, et al. 2015. The p53-induced lincRNA-p21 derails somatic cell reprogramming by sustaining H3K9me3 and CpG methylation at pluripotency gene promoters. Cell Res. 25, 80–92. ( 10.1038/cr.2014.165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JY, Sirey T, Honti F, Graham B, Piovesan A, Merkenschlager M, Webber C, Ponting CP, Marques AC. 2015. Extensive microRNA-mediated crosstalk between lncRNAs and mRNAs in mouse embryonic stem cells. Genome Res. 25, 655–666. ( 10.1101/gr.181974.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swiezewski S, Liu F, Magusin A, Dean C. 2009. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462, 799–802. ( 10.1038/nature08618) [DOI] [PubMed] [Google Scholar]
- 11.Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. 2014. Novel insights on interactions between folate and lipid metabolism. Biofactors 40, 277–283. ( 10.1002/biof.1154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. 2009. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. Br. Med. J. 338, b1673 ( 10.1136/bmj.b1673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. 2013. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 4, 2889 ( 10.1038/ncomms3889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Geng G, Li Q, Sun X, Cao H, Liu Y. 2014. Effects of alginate on frozen-thawed boar spermatozoa quality, lipid peroxidation and antioxidant enzymes activities. Anim. Reprod. Sci. 147, 112–118. ( 10.1016/j.anireprosci.2014.04.007) [DOI] [PubMed] [Google Scholar]
- 15.Naghi SA, Ghasemi HA, Taherpour K. 2017. Evaluation of Aloe vera and synbiotic as antibiotic growth promoter substitutions on performance, gut morphology, immune responses and blood constitutes of broiler chickens. Anim. Sci. J. 88, 306–313. ( 10.1111/asj.12629) [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Shen J, Pu X, Zheng N, Deng Z, Zhang Z, Li H. 2017. Urinary time- or dose-dependent metabolic biomarkers of aristolochic acid-induced nephrotoxicity in rats. Toxicol. Sci. 156, 123–132. ( 10.1093/toxsci/kfw244) [DOI] [PubMed] [Google Scholar]
- 17.Zhao W, He X, Hoadley KA, Parker JS, Hayes DN, Perou CM. 2014. Comparison of RNA-Seq by poly (A) capture, ribosomal RNA depletion, and DNA microarray for expression profiling. BMC Genomics 15, 419 ( 10.1186/1471-2164-15-419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, et al. 2018. Identification and characterization of long noncoding RNAs and mRNAs expression profiles related to postnatal liver maturation of breeder roosters using ribo-zero RNA sequencing. BMC Genomics 19, 498 ( 10.1186/s12864-018-4891-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Ren X, Li Y, Guo W, Lei X, Yao J, Yang X. 2017. Effect of dietary Astragalus polysaccharide supplements on testicular miRNA expression profiles and enzymatic changes of breeder cocks. Sci. Rep. 6, 38864 ( 10.1038/srep38864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie C, et al. 2011. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39, W316–W322. ( 10.1093/nar/gkr483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Yan J, Yuan X, Yang R, Dan T, Wang X, Kong G, Gao S. 2016. A computationally constructed ceRNA interaction network based on a comparison of the SHEE and SHEEC cell lines. Cell. Mol. Biol. Lett. 21, 21 ( 10.1186/s11658-016-0022-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Shen J, Yang X, Sun Q, Yang X. 2018. Folic acid reduced triglycerides deposition in primary chicken hepatocytes. J. Agric. Food Chem. 66, 13 162–13 172. ( 10.1021/acs.jafc.8b05193) [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 24.Boxmeer JC, et al. 2009. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil. Steril. 92, 548–556. ( 10.1016/j.fertnstert.2008.06.010) [DOI] [PubMed] [Google Scholar]
- 25.Williams AC, Ford WC. 2004. Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm. Biol. Reprod. 71, 1309–1316. ( 10.1095/biolreprod.104.028407) [DOI] [PubMed] [Google Scholar]
- 26.Christensen KE, et al. 2015. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 101, 646–658. ( 10.3945/ajcn.114.086603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen KE, et al. 2016. Moderate folic acid supplementation and mthfd1-synthetase deficiency in mice, a model for the r653q variant, result in embryonic defects and abnormal placental development. Am. J. Clin. Nutr. 104, 1459–1469. ( 10.3945/ajcn.116.139519) [DOI] [PubMed] [Google Scholar]
- 28.Stark R, et al. 2014. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J. Biol. Chem. 289, 7257–7263. ( 10.1074/jbc.C113.544759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, et al. 2013. Thyroid hormone-responsive spot 14 homolog promotes hepatic lipogenesis, and its expression is regulated by liver x receptor α through a sterol regulatory element-binding protein 1c–dependent mechanism in mice. Hepatology 58, 617–628. ( 10.1002/hep.26272) [DOI] [PubMed] [Google Scholar]
- 30.La Paglia L, Listì A, Caruso S, Amodeo V, Passiglia F, Bazan V, Fanale D.. 2017. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. PPAR Res. 2017, 8187235 ( 10.1155/2017/8187235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. 2013. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566. ( 10.1038/nm.3159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haeusler RA, et al. 2014. Integrated control of hepatic lipogenesis vs. glucose production requires foxo transcription factors. Nat. Commun. 5, 5190 ( 10.1038/ncomms6190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers AB, Morgan CP, Leu NA, Bale TL. 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl Acad. Sci. USA 112, 13 699–13 704. ( 10.1073/pnas.1508347112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669. ( 10.1038/nn.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang KC, Chang HY. 2011. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 43, 904–914. ( 10.1016/j.molcel.2011.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devanapally S, Ravikumar S, Jose AM. 2015. Double-stranded RNA made in C. elegans neurons can enter the germline and cause transgenerational gene silencing. Proc. Natl Acad. Sci. USA 112, 2133–2138. ( 10.1073/pnas.1423333112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay Y, Rinn J, Pandolfi PP. 2014. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352. ( 10.1038/nature12986) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data have been deposited in Gene Expression Omnibus and can be accessed with the number GSE122759.