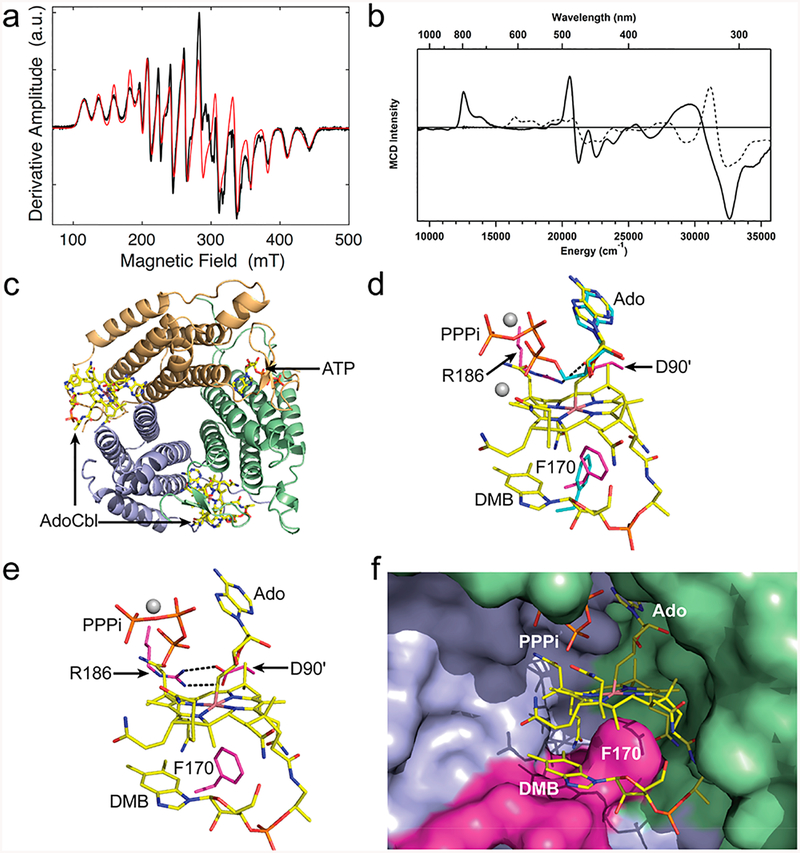
Figure 2.
Spectroscopic and structural characterization of B12 bound to ATR. (a) EPR spectrum of 4C cob(II)alamin formed after addition of PPPi under aerobic conditions (black). The red line corresponds to the simulated spectrum. (b) MCD spectra of 4C cob(II)alamin (solid line) generated under the same conditions as (a) and of 5C cob(II)inamide (dashed lines) as a reference. (c) Structure of human ATR showing two subunits occupied by AdoCbl and a third by ATP.(d) Superposition of the adenosine rings in ATP (cyan, PDB: 21DX) and AdoCbl (yellow, PDB: 6D5K, this study) bound to ATR. (e) Close-up of the ATR·AdoCbl·PPPi active site structure (PDB: 6D5X, this study). (f) Close-up of human ATR showing that the DMB tail, tucked below the corrin ring, is kept from coordinating to the cobalt by Phe170 on a hydrophobic loop (pink). Adjacent subunits that form the active site are in blue and green. The gray spheres in panels d and e represent magnesium.