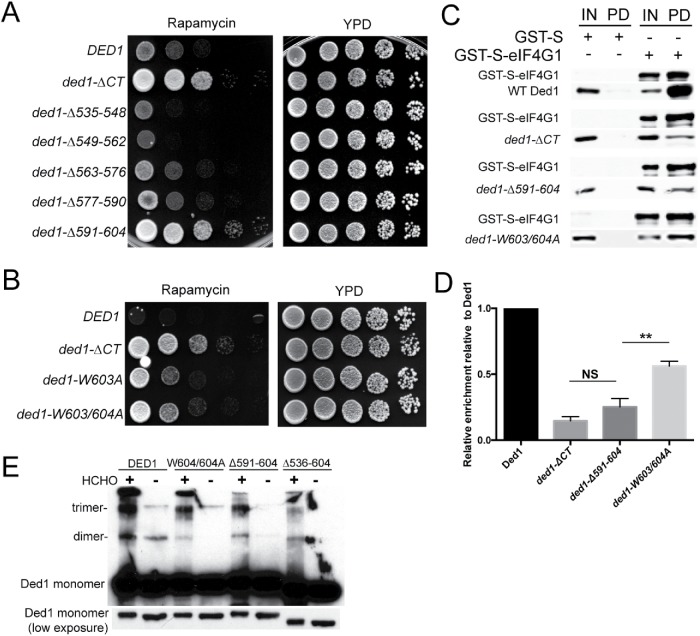
FIGURE 6:
Conserved critical residues in the Ded1 C-terminus are required for its function in stress, interaction with eIF4G1, and efficient oligomerization. (A) Cells containing DED1, ded1-∆CT, or one of a series of 14 amino acid C-terminal deletions were serially diluted onto rich media ± rapamycin (200 ng/ml) and incubated at 30°C. (B) DED1, ded1-∆CT, ded1-W604A, or ded1-W603/604A cells were serially diluted onto rich media ± rapamycin (200 ng/ml), and incubated at 30°C. (C) Bacterially expressed GST-S-eIF4G1 or GST-S was enriched using a glutathione sepharose resin, and affinity chromatography was performed with the addition of recombinant Ded1, ded1-∆CT, ded1-∆591-604, or ded1W603/604A. The bound proteins were analyzed via Western blotting using antibodies to Ded1 and eIF4G1. Input (IN), pull down (PD). (D) Quantitation of the results in C. The data show the mean and SEM of three independent experiments. **p < 0.01 vs. ded1-∆591-604. (E) Recombinant Ded1, ded1-∆CT, ded1-∆591-604, and ded1-W603/604A were cross-linked with formaldehyde or left untreated and visualized via Western blotting with anti-Ded1 antibody. Mobilities of the Ded1 monomer, dimer and trimer are indicated. Note that a lower exposure of Ded1 monomer is also shown to demonstrate equal protein loading.