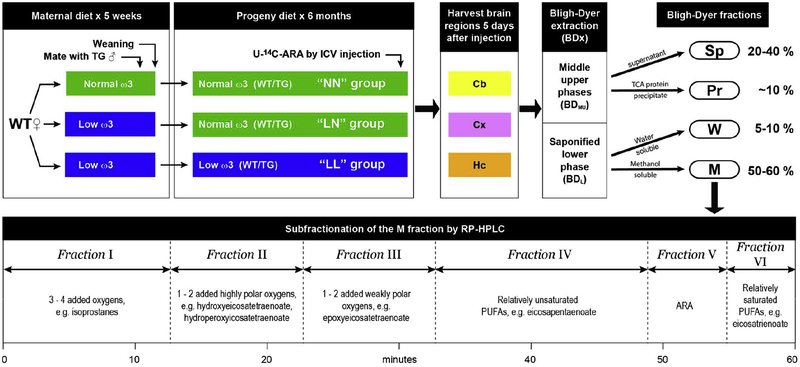
Figure 1. Experimental scheme.
There were 5 stages in the experimental scheme. Stage 1: Female WT mice, fed either an ω3-replete or an ω3-deficient diet for 5 weeks, were mated with hemizygous male TG mice. Stage 2: Upon weaning, the progeny of female mice on an ω3-deficient diet were either continued on an ω3-deficient diet or switched to a ω3-replete diet for 6 months, while the progeny of female mice on an ω3-replete diet were continued on an ω3-replete diet for 6 months. This protocol yielded WT and TG mice in 3 different “diet groups” designated NN, LN, and LL. Five days before brain tissue was harvested, U-14C-ARA was introduced by ICV injection. Stage 3: Three brain regions from each mouse were dissected, extracted, and fractionated as described in Experimental Methods. Cb – cerebellum; Cx – cortex; Hc–hippocampus; BDMU – middle/upper phase of the Bligh Dyer extract; BDL – lower phase of the Bligh Dyer extraction. Stage 4: The middle/upper and the lower phases of the Bligh Dyer extract were fractionated according to solubility. Sp – protein-depleted supernatant from the BDMU phase; Pr – Proteins precipitated from the BDMU by TCA; W – water-soluble fraction from the BDL phase; M – methanol-soluble fraction from the BDL phase. Stage 6: The M fraction was subfractionated by reversed-phase (RP) HPLC. Typical products identified by mass spectrometry in each subfraction are indicated.