Abstract
Background
Plasma gelsolin (pGSN) is an abundant circulating protein that neutralizes actin exposed by damaged cells, modulates inflammatory responses, and enhances alveolar macrophage antimicrobial activity. We investigated whether adults with low pGSN at hospital admission for community-acquired pneumonia (CAP) were at high risk for severe outcomes.
Methods
Admission pGSN concentrations in 455 adults hospitalized with CAP were measured using enzyme-linked immunosorbent assay. Patients were grouped into the following 4 hierarchical, mutually exclusive categories based on maximum clinical severity experienced during their hospitalization: general floor care without intensive care unit (ICU) admission, invasive respiratory or vasopressor support (IRVS), or death; ICU care without IRVS or death; IRVS without death; or death. Admission pGSN concentrations were compared across these discrete outcome categories. Additionally, outcomes among patients in the lowest quartile of pGSN concentration were compared to those in the upper 3 quartiles.
Results
Overall, median (interquartile range) pGSN concentration was 38.1 (32.1, 45.7) μg/mL. Patients with more severe outcomes had lower pGSN concentrations (P = .0001); median values were 40.3 μg/mL for floor patients, 36.7 μg/mL for ICU patients, 36.5 μg/mL for patients receiving IRVS, and 25.7 μg/mL for patients who died. Compared to patients with higher pGSN concentrations, patients in the lowest quartile (pGSN ≤ 32.1 μg/mL) more often required IRVS (21.2% vs 11.7%, P = .0114) and died (8.8% vs 0.9%, P < .0001).
Conclusions
Among adults hospitalized with CAP, lower pGSN concentrations were associated with more severe clinical outcomes. Future studies are planned to investigate possible therapeutic benefits of recombinant human pGSN in this population.
Keywords: pneumonia, plasma gelsolin, antimicrobial resistance
Plasma gelsolin (pGSN) is a component of the innate immune system. Adults hospitalized with pneumonia with the greatest pGSN depletion had the highest risk of severe clinical outcomes, highlighting the potential role of pGSN in the pathophysiology of severe pneumonia.
Community-acquired pneumonia (CAP) commonly results in hospitalization and remains a leading cause of morbidity and mortality worldwide [1, 2]. Patients with pneumonia are at risk for death despite optimal antibiotics and supportive care [1–4]. Pathogens that cause pneumonia are frequently unknown even after extensive etiologic testing [3]. Furthermore, development of antibiotic resistance among CAP pathogens continues to be a concern [5]. Therefore, the development of adjunctive host-based antiinflammatory therapies to bolster antimicrobial treatment of pneumonia has emerged as a high priority [6].
Plasma gelsolin (pGSN) is an abundant circulating protein that functions as a regulatory component of the innate immune system [7, 8]. In addition to binding and severing actin filaments leaked from damaged tissues [9–14], pGSN complexes with proinflammatory lipid and peptide mediators with high affinity and enhances bacterial uptake and killing by resident macrophages [13–17]. Prior studies suggest pGSN also inactivates bacterial products (eg, bacterial endotoxin), while enhancing the actions of endogenous antibacterial peptides (eg, LL-37) and certain cationic antibiotics (eg, aminoglycosides) [18–21]. During inflammation and cellular injury, the concentration of circulating pGSN drops as it binds to actin locally at the site of injury [7, 11, 22–34]. Greater depletion of pGSN in the circulation is associated with increased risk of mortality and other severe complications in several systemic inflammatory illnesses [7, 11, 22–35]. Therefore, pGSN is an attractive candidate for development as a therapeutic antiinflammatory agent for serious infections [8]. In animal models, pGSN has demonstrated potential therapeutic benefit [14, 36, 37]. Clinical trials to evaluate recombinant human pGSN as adjunctive therapy in severe infections are being planned.
Our objective in this study was to investigate the association of pGSN depletion with severe short-term clinical outcomes in a well-characterized cohort of adults hospitalized with CAP to inform the conduct of future pGSN trials. Specifically, we determined whether patients who present with low circulating pGSN concentrations were at high risk for death, septic shock, and respiratory failure when treated with standard current therapy in order to understand if pGSN depletion identified a high-risk CAP population that may potentially benefit from exogenous pGSN supplementation.
METHODS
This observational study was nested within the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a prospective, multicenter, active surveillance study of adults hospitalized with CAP recruited from January 2010 through June 2012 [3]. Institutional review boards at each participating hospital and the CDC approved the study. Written informed consent was obtained from each participant or authorized representative.
Study Population
The EPIC study cohort has been previously described [3]. In brief, patients hospitalized with CAP were enrolled at 5 hospitals, including 2 in Nashville, Tennessee, and 3 in Chicago, Illinois. Adults (aged ≥ 18 years) were eligible if they were admitted to a study hospital, resided in the study catchment area, exhibited clinical evidence of an acute respiratory infection, and had radiographic evidence of pneumonia. Patients with any of the following criteria were excluded: recent hospitalization (<28 days for immunocompetent patients and <90 days for immunosuppressed patients), nursing home resident not functionally independent, tracheotomy, gastrostomy, cystic fibrosis, cancer with neutropenia, solid organ or hematopoietic stem-cell transplant within the previous 90 days, active graft-versus-host disease, bronchiolitis obliterans, or human immunodeficiency virus infection with a CD4 cell count <200 mm3. Patients were treated according to usual care by clinical teams independent of the study protocol.
Plasma was collected from enrolled patients at the time of hospital admission and stored at –80°C. The population for the current study consisted of all adult patients enrolled in the EPIC study who had a sufficient volume (≥50 μL) of banked plasma to measure pGSN concentrations after completion of the primary laboratory tests for the EPIC study.
pGSN Measurements
Frozen plasma specimens were shipped from enrolling hospitals to a central laboratory for pGSN measurement by BioAegis Therapeutics, Inc (North Brunswick, NJ) using a proprietary enzyme-link immunosorbent assay (ELISA). Laboratory personnel who performed the assay were blinded to clinical outcomes. The pGSN results were not available for patient management.
A rabbit polyclonal antibody developed against 16 amino acids specific to the N-terminus of human pGSN was utilized as the capture antibody for pGSN in plasma specimens and a recombinant human pGSN standard. Bound gelsolin was detected with a commercially available anti-gelsolin antibody (Sigma clone 2C4) followed by horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG). After a 1-hour incubation, the ELISA plate was washed to remove unbound material. The amount of bound HRP-conjugated anti-mouse IgG antibody (Jackson Immunoresearch Laboratories) was detected by addition of tetramethylbenzidine. The optical density of the resultant color was directly proportional to the amount of gelsolin in the initial specimen determined by a standard curve constructed with rhu-pGSN reference standards as well as a plasma standard with a known amount of endogenous pGSN.
The lower limit of pGSN detection by this ELISA was approximately 10 μg/mL, with a dynamic linear range up to approximately 100 μg/mL. In addition to testing pneumonia patients enrolled in this study, pGSN concentration was also measured using the same method in plasma samples from 20 healthy adult humans (normal single donor human plasma samples purchased from Innovative Research). Median pGSN concentration in these control samples from healthy adults was 56.8 μg/mL (interquartile range [IQR], 52.6–65.4 μg/mL).
Pathogen Testing
As previously described [3] and detailed in the Supplementary Materials, each enrolled patient underwent systematic pathogen testing per study protocol. For the current analysis, patients were classified into the following 3 mutually exclusive categories based on results of pathogen testing: only viral pathogens detected, bacterial pathogens (including those with viral codetections, mycobacteria, or Pneumocystis) detected, and no pathogen detected.
Outcomes
EPIC study personnel at each of the 5 enrolling sites conducted standardized patient interviews and medical record reviews to collect demographic and clinical data. Pneumonia severity at hospital admission was assessed using the Pneumonia Severity Index [38].
Clinical outcomes included intensive care unit (ICU) care, invasive respiratory or vasopressor support (IRVS), and in-hospital death. ICU care was defined as treatment in an ICU at any time during the index hospitalization for CAP, including both patients initially admitted to an ICU and those initially admitted to a general floor but later transferred to an ICU. IRVS was defined as initiation of invasive mechanical ventilation through an endotracheal tube or tracheostomy for respiratory failure or vasopressor administration for septic shock within 72 hours of admission [39, 40]. In-hospital death was defined as death due to any cause during the index hospitalization for CAP.
These outcomes were used to construct a 4-level ordinal scale to describe clinical outcomes experienced by patients during hospitalization. Patients were classified in mutually exclusive categories according to the most severe category that they fulfilled as follows: (1) general floor care without ICU care, IRVS, or death; (2) ICU care without IRVS or death; (3) IRVS without death; and (4) death.
Statistical Analyses
The distributions of pGSN concentration were compared across the 4-level ordinal outcome scale with the Kruskal-Wallis test. Outcome severity was also dichotomized as low severity (categories 1–2: no IRVS or death) vs high severity (categories 3–4: IRVS or death); pGSN concentrations were compared between these 2 groups with the Wilcoxon rank sum test.
The study population was divided into quartiles based on admission pGSN concentrations. The proportion of patients who experienced any of the severe outcomes (ICU care, IRVS, and death) was examined across quartiles, with the lowest pGSN quartile compared to the other 3 quartiles using the χ2 test.
We also evaluated the association of admission pGSN concentrations on a continuous scale with the risk of IRVS or death (severity categories 3–4). For this analysis, pGSN values were modeled with a restricted cubic spline function with 4 knots located at the 5th, 35th, 65th, and 95th percentile of pGSN concentration in the study population [41]. A logistic regression model was constructed with pGSN splines as the independent variable and the composite of IRVS or death as the dependent variable. Predicted probabilities from this model were used to estimate the risk of IRVS or death according to admission pGSN concentration.
We assessed pGSN distribution by pathogen type detected (only viral, bacterial ± viral codetection, and none). The pGSN concentration in the bacterial group was compared to the concentration in the viral group using the Wilcoxon rank sum test.
RESULTS
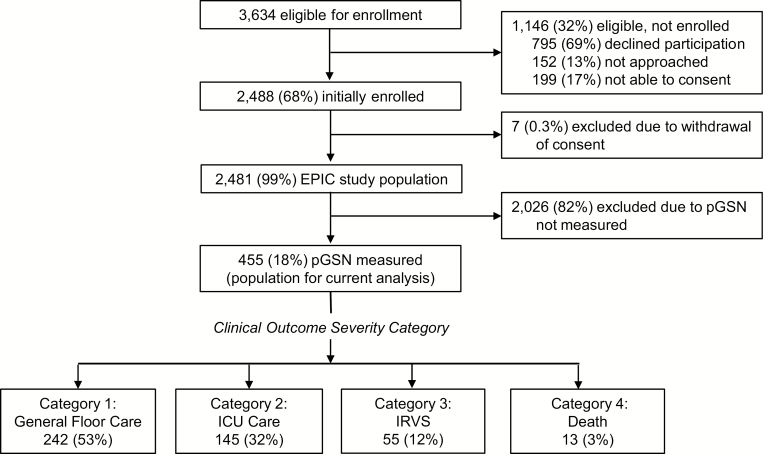
Among 2481 adults in the EPIC study, 455 participants (18%) had an adequate plasma volume for pGSN measurement and were included in this study (Figure 1). For the 455 analyzed patients, median age was 58 years, 53% were white, and asthma and chronic obstructive pulmonary disease were the most common comorbidities (Table 1). Demographics and comorbidities were similar for patients with pGSN measurements and those in the EPIC study without adequate banked plasma volume for pGSN measurements (Supplementary Table 1). However, patients who received ICU care were more likely to have large sample volumes collected; consequently, ICU care and IRVS were overrepresented in patients undergoing pGSN measurements (Supplementary Table 2).
Figure 1.
Flow diagram of patient inclusion in the nested analysis.
Table 1.
Baseline Patient Characteristics
| Characteristic | Adults Hospitalized With Community-acquired Pneumonia and Plasma Gelsolin Results (n = 455) |
|---|---|
| Age, median years (interquartile range) | 58 (47, 70) |
| Female sex, n (%) | 231 (50.8) |
| Race and ethnicity, n (%) | |
| Non-Hispanic white | 241 (53.0) |
| Non-Hispanic black | 157 (34.5) |
| Hispanic | 37 (8.1) |
| Other | 20 (4.4) |
| Age groups, years, n (%) | |
| 18–44 | 101 (22.2) |
| 45–64 | 202 (44.4) |
| 65–79 | 92 (20.2) |
| ≥80 | 60 (13.2) |
| Chronic medical conditions, n (%) | |
| Current smoker | 131 (28.8) |
| Asthma | 119 (26.2) |
| Chronic obstructive lung disease | 117 (25.7) |
| Diabetes mellitus | 111 (24.4) |
| Immunosuppression | 100 (22.0) |
| Cancer | 96 (21.1) |
| Chronic heart failure | 81 (17.8) |
| Chronic kidney disease | 76 (16.7) |
| Chronic liver disease | 26 (5.7) |
| Human immunodeficiency virus infection | 10 (2.2) |
| Pneumonia severity index risk class [38] | |
| I | 81 (17.8) |
| II | 103 (22.6) |
| III | 89 (19.6) |
| IV | 128 (28.1) |
| V | 54 (11.9) |
| Antibiotics before hospital presentation | 97 (21.3) |
Clinical Outcomes
Thirteen (2.9%) patients died during hospitalization (severity category 4; Table 2). An additional 55 (12.1%) patients had IRVS without death (severity category 3), resulting in 68 (14.9%) patients with the composite severe outcome of death or IRVS (severity categories 3–4). ICU care without death or IRVS occurred in 145 (31.9%) patients (severity category 2). The remaining 242 (53.2%) patients were admitted to a hospital floor and did not experience death, IRVS, or ICU care (severity category 1).
Table 2.
Ordinal Scale to Describe Clinical Outcome Severity Among Adults Hospitalized With Community-acquired Pneumonia
| Severity Category | Description of Outcomes | n (%)in Each Category | Cumulative n (%) in Each Category Plus More Severe Categories |
|---|---|---|---|
| 4 | Death | 13 (2.9) | 13 (2.9) |
| 3 | Invasive respiratory or vasopressor support | 55 (12.1) | 68 (14.9) |
| 2 | Intensive care unit care | 145 (31.9) | 213 (46.8) |
| 1 | General hospital floor care only | 242 (53.2) | 455 (100) |
Outcomes were ascertained during the hospitalization for community-acquired pneumonia, with censoring at the time of hospital discharge.
pGSN concentrations
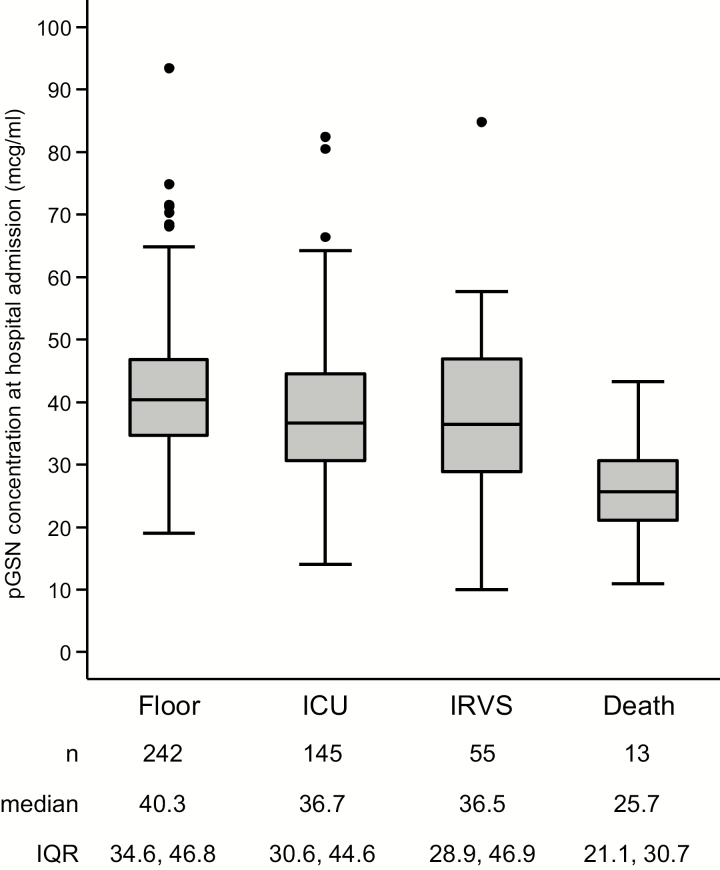
Median pGSN concentration at hospital admission in the study population was 38.1 μg/mL (IQR, 32.1–45.7 μg/mL), with a full range from 9.9 to 93.4 μg/mL (Supplementary Figure 1). Using the 4-level outcomes scale, patients in more severe outcome categories had lower pGSN concentrations (P = .0001; Figure 2). Similarly, after dichotomizing patient outcomes, patients who experienced IRVS or death (severity categories 3–4) had lower pGSN concentrations than those without IRVS or death (severity categories 1–2; median 34.8 vs 39.3 μg/mL; P = .0013). pGSN concentrations stratified by pneumonia severity categories at hospital presentation as defined by several clinical scoring systems are displayed in Supplementary Figures 2–7.
Figure 2.
Box plot of plasma gelsolin concentration by the following 4-category ordinal scale of clinical outcome severity: general hospital floor care only, intensive care unit care, IRVS, and in-hospital death. The central horizontal line of each box plot represents the median, with the box denoting the interquartile range (IQR), the whiskers representing 1.5 times the IQR, and dots showing outliers beyond the whiskers. Abbreviations: ICU, intensive care unit; IQR, interquartile range; IRVS, invasive respiratory or vasopressor support; pGSN, plasma gelsolin.
Severe Hospital Outcomes According to Admission pGSN Concentration
Compared to patients with pGSN concentration in the upper 3 quartiles for the study population, patients with pGSN concentration in the lowest quartile (pGSN ≤ 32.1 μg/mL) were more likely to experience each of the severe clinical outcomes, including death, IRVS, and ICU care (Table 3).
Table 3.
Number and Percentage of Patients Who Experienced Severe Clinical Outcomes, by Plasma Gelsolin Quartiles
| Plasma Gelsolin Quartile(n [Column %]) | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1(≤32.1 μg/mL) | Quartile 2(32.2–38.1 μg/mL) | Quartile 3(38.2–45.7 μg/mL) | Quartile 4(≥45.8 μg/mL) | Quartiles 2–4(≥32.2 μg/mL) | P Value(Quartile 1 vs Quartiles 2–4) | ||
| Outcome | (n = 113) | (n = 114) | (n = 114) | (n = 114) | (n = 342) | ||
| Outcome severity category | |||||||
| Category 4 (death) | 10 (8.8) | 0 | 3 (2.6) | 0 | 3 (0.9) | <.0001 | |
| Category 3 (IRVS) or category 4 (death) | 28 (24.8) | 12 (10.5) | 14 (12.3) | 14 (12.3) | 40 (11.7) | .0007 | |
| Category 2 (ICU), category 3 (IRVS), or category 4 (death) | 73 (64.6) | 50 (43.9) | 46 (40.4) | 44 (38.6) | 140 (40.9) | <.0001 | |
| Discrete outcomes | |||||||
| IRVS | 24 (21.2) | 12 (10.5) | 14 (12.3) | 14 (12.3) | 40 (11.7) | .0114 | |
| Invasive respiratory support | 19 (16.8) | 11 (9.6) | 9 (7.9) | 10 (8.8) | 30 (8.8) | .0168 | |
| Vasopressor support | 16 (14.2) | 5 (4.4) | 7 (6.1) | 9 (7.9) | 21 (6.1) | .0069 | |
| ICU care | 72 (63.7) | 50 (43.9) | 46 (40.4) | 44 (38.6) | 140 (40.9) | <.0001 | |
| Bacterial pathogen detected | 29 (25.7) | 13 (11.4) | 16 (14.0) | 8 (7.0) | 37 (10.8) | .0001 | |
Abbreviations: ICU, intensive care unit; IRVS, invasive respiratory or vasopressor support.
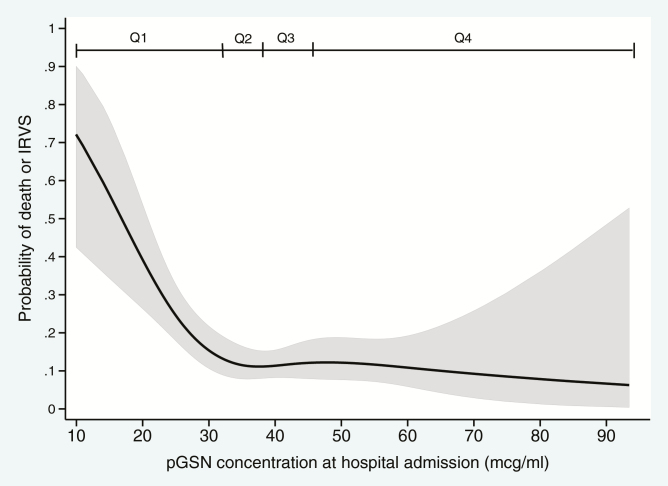
When pGSN was evaluated on a continuous scale, the risk of death or IRVS was highest at very low pGSN concentrations, with declining risk as pGSN increased through the lowest quartile of pGSN values and a plateau of risk for patients with pGSN higher than the lowest quartile (Figure 3). For example, the risk of death or IRVS was estimated at 0.72 (95% confidence interval [CI], .64–.95) for patients with a pGSN concentration of 10.0 μg/mL and at 0.14 (95% CI, .10–.20) for patients with a pGSN concentration of 32.1 μg/mL. The estimated risk of death or IRVS ranged from 0.07 to 0.13 for patients with pGSN concentrations greater than 32.1 μg/mL.
Figure 3.
Probability for the composite of death or IRVS (severity categories, 3–4) according to plasma gelsolin (pGSN) concentration. The shaded area represents the 95% confidence interval band. Quartiles of pGSN concentration are denoted with Q1–Q4. Abbreviations: IRVS, invasive respiratory or vasopressor support; pGSN, plasma gelsolin.
Admission pGSN Concentration by Pathogen Type
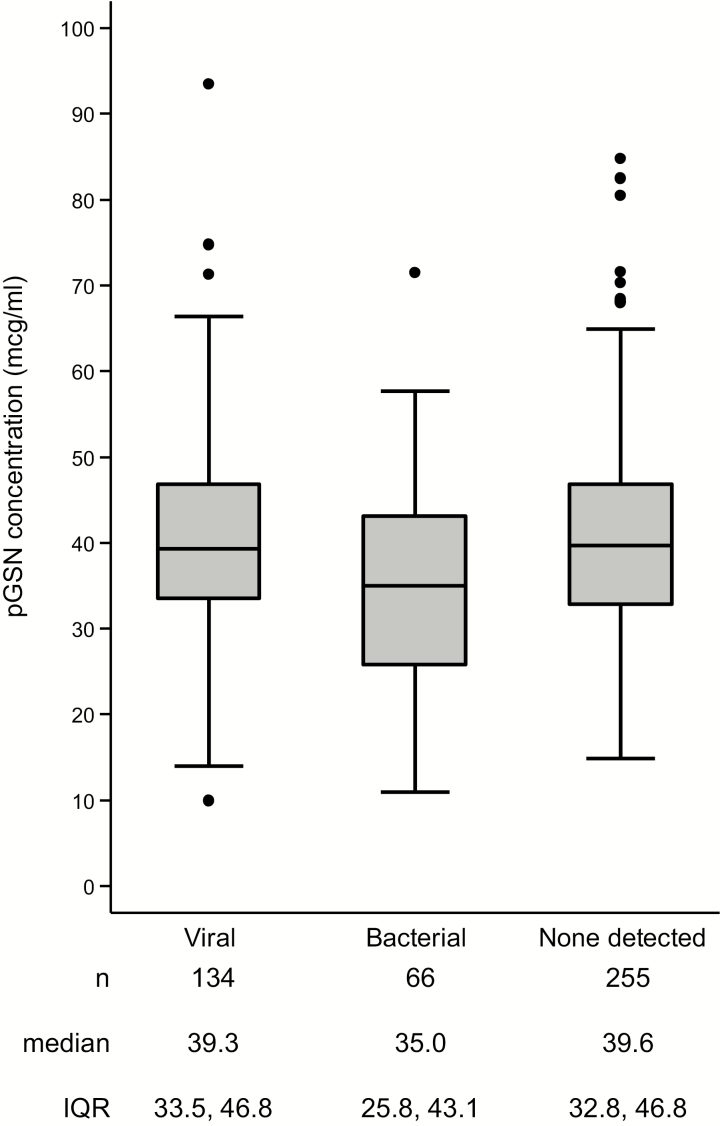
A total of 134 (29.5%) patients were classified as having viral pneumonia based on detection of a viral pathogen without codetection of a bacterial pathogen. A total of 66 (14.5%) patients were classified in the bacterial group, including 17 with codetection of bacterial and viral pathogens, 2 with mycobacteria, and 1 with Pneumocystis. The remaining 255 (56.0%) patients did not have a pathogen detected despite extensive investigation (Figure 4).
Figure 4.
Box plot of plasma gelsolin concentration by the following detected pathogen type: viral, bacterial, and no pathogen detected. The central horizontal line of each box plot represents the median, with the box denoting the interquartile range (IQR), the whiskers representing 1.5 times the IQR, and dots showing outliers beyond the whiskers. Abbreviations: IQR, interquartile range; pGSN, plasma gelsolin.
Prevalence of a bacterial pathogen was 25.7% for patients in the lowest quartile of pGSN concentration compared to 10.8% for patients in the higher 3 quartiles of pGSN concentration (P = .0001; Table 3).
DISCUSSION
In this observational study of adults hospitalized for CAP, low circulating pGSN concentration at hospital admission was associated with higher risk of severe, short-term clinical outcomes. In this study of 455 patients, those with pGSN concentration in the lowest quartile for the study population had approximately 9 times higher risk of death, 2 times higher risk of septic shock requiring vasopressors, and 2 times higher risk of respiratory failure requiring invasive mechanical ventilation compared to patients with higher pGSN. Lower pGSN also appeared to be more common in bacterial pneumonia than in pneumonia without a bacterial pathogen detected.
Recombinant human pGSN has been proposed as a potential adjunctive therapy for severe infections [7, 8, 14, 36, 37]. The current study demonstrates that admission pGSN concentrations vary widely in adults hospitalized with CAP, adverse clinical outcomes are common in this population, and patients with the lowest pGSN concentrations are at the highest risk for these adverse outcomes. These data suggest that adults hospitalized with CAP, especially those with low circulating pGSN concentrations, constitute an important patient population to consider for future trials to evaluate the potential therapeutic effects of recombinant human pGSN supplementation.
The current study adds to the growing literature on pGSN, including its depletion in severe acute infections. Cellular injury, such as that caused by severe infection, releases actin from the intracellular compartment into the surrounding tissue, resulting in an inflammatory response [7, 8, 12]. Prior studies have demonstrated that pGSN binds and degrades extracellular actin as part of the “extracellular actin scavenger system” and augments the antimicrobial activity of resident macrophages, enhancing local uptake and killing of pathogens [7, 8, 13, 17]. Additionally, pGSN remaining in the circulation unbound to actin dampens systemic inflammation by binding proinflammatory mediators, such as platelet-activating factor, fibronectin, fibrin peptides, and lysophosphatidic acid [13–17]. With larger cellular insults, more actin is exposed at the site of injury, resulting in more pGSN binding to actin locally and depletion of pGSN in the circulation. Lower levels of circulating pGSN hamper the host’s ability to limit systemic inflammation [8]. Thus, low concentrations of circulating pGSN may contribute to uncontrolled systemic inflammation.
Consistent with this paradigm, prior human observational studies have demonstrated an association between low circulating pGSN concentration and increased risk for severe outcomes in several acute inflammatory illnesses, including sepsis, major trauma, burns, and acute lung and liver injury [11, 22–25, 29–32]. The current study demonstrates a similar pattern in CAP. It is not known whether repletion of pGSN in patients with low circulating pGSN concentrations improves clinical outcomes, and this will be the focus of future trials. In animal models of severe infection, pGSN repletion does appear to have potential therapeutic benefit [14, 36, 37]. For example, in a murine model of highly lethal pneumococcal pneumonia, the administration of recombinant human (rhu)-pGSN improved survival even in the absence of antibiotic therapy [37]. In future human trials, low pGSN concentration may be used to enrich study populations for high-risk patients who have pathophysiology well suited for testing the potential therapeutic effects of pGSN infusion.
The strengths of our study included its multicenter design, a consistent case definition for CAP including radiographic confirmation, consistent methods for ascertaining outcomes and obtaining archival plasma samples that were used at all sites and developed a priori, and systematic protocol-driven pathogen testing.
Our study also had limitations. First, the study population was a convenience sample of patients enrolled in the parent EPIC study [3] with residual banked plasma specimens for pGSN testing. Second, pGSN was only measured in specimens collected at hospital admission; therefore, changes in pGSN during clinical deterioration and recovery after admission could not be assessed. Third, while a substantial number of pneumonia-associated deaths occur after hospital discharge [1], only in-hospital outcomes were assessed in this study. Fourth, because the EPIC study had lower in-hospital mortality than some other recent CAP studies [1], results of this study may not be directly generalizable to CAP populations with higher mortality. Fifth, pGSN concentration is technically difficult to measure and different assays can lead to variable results [35]. For all patients in this study, we consistently used the same immunoassay and laboratory techniques at a central laboratory with expertise in pGSN measurement. Last, we evaluated the bivariate association of pGSN and clinical outcomes without adjustment for other potential predictors because we are not proposing pGSN as a biomarker for use in clinical medicine. Rather, our data support the exploration of pGSN concentration as a biomarker for prognostic and predictive enrichment in future trials to evaluate recombinant pGSN as a therapeutic agent.
In conclusion, adults hospitalized with CAP who have low endogenous circulating pGSN levels are at increased risk for severe short-term outcomes, including respiratory failure, shock, and death. These findings suggest a potential role of pGSN depletion in the pathophysiology of severe CAP and highlight that adults hospitalized with CAP and low endogenous pGSN may be an important population to target in future trials to evaluate recombinant human pGSN as a therapeutic. Additional work is needed to understand changes in pGSN concentration during the course of severe infection and in response to infection by specific pathogens.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Edward Kowalik and Jeremy Pronchik from BioAegis Therapeutics, Inc for planning and executing the enzyme-link immunosorbent assay measurements of plasma gelsolin (pGSN) concentrations as well as reviewing the manuscript at several stages. BioAegis Therapeutics provided materials and funds for measurement of plasma gelsolin in this study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by a cooperative agreement with the CDC (U18 IP000299). Scientists from the CDC are included as authors. Materials and funds for pGSN measurement were provided by BioAegis Therapeutics, Inc. Scientists from BioAegis Therapeutics, Inc are included as authors. W. H. S. was supported in part by the National Institute of General Medical Sciences (K23GM110469). C. G. G. was supported in part by the National Institute on Aging (R01AG043471).
Potential conflicts of interest. M. J. D., T. P. S., and S. L. L. are paid members of BioAegis Therapeutics, Inc. W. H. S. reports personal fees from Cempra Pharmaceuticals, Ferring Pharmaceuticals, BioTest AG, Gilead Pharmaceuticals, Pfizer, and Merck outside the submitted work. E. J. A. reports personal fees from AbbVie and grants from Novavax, Pfizer, Merck, MedImmune, and Sanofi Pasteur outside the submitted work. All remaining authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ramirez JA, Wiemken TL, Peyrani P, et al. . University of Louisville Pneumonia Study: adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65: 1806–12. [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Study. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17: 1133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain S, Self WH, Wunderink RG, et al. . Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med 2015; 373: 415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mortensen EM, Coley CM, Singer DE, et al. . Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team Cohort Study. Arch Intern Med 2002; 162:1059–64. [DOI] [PubMed] [Google Scholar]
- 5. Ho PL, Cheng VC, Chu CM. Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii. Chest 2009; 136:1119–27. [DOI] [PubMed] [Google Scholar]
- 6. Wunderink RG, Mandell L. Adjunctive therapy in community-acquired pneumonia. Semin Respir Crit Care Med 2012; 33:311–8. [DOI] [PubMed] [Google Scholar]
- 7. Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med 1992; 326:1335–41. [DOI] [PubMed] [Google Scholar]
- 8. DiNubile MJ. Plasma gelsolin: in search of its raison d’etre. Am J Physiol Cell Physiol 2007; 292: C1240–2. [DOI] [PubMed] [Google Scholar]
- 9. Hartwig JH, Chambers KA, Stossel TP. Association of gelsolin with actin filaments and cell membranes of macrophages and platelets. J Cell Biol 1989; 108:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasconcellos CA, Lind SE. Coordinated inhibition of actin-induced platelet aggregation by plasma gelsolin and vitamin D-binding protein. Blood 1993; 82:3648–57. [PubMed] [Google Scholar]
- 11. Smith DB, Janmey PA, Sherwood JA, Howard RJ, Lind SE. Decreased plasma gelsolin levels in patients with Plasmodium falciparum malaria: a consequence of hemolysis? Blood 1988; 72:214–8. [PubMed] [Google Scholar]
- 12. Erukhimov JA, Tang ZL, Johnson BA, et al. . Actin-containing sera from patients with adult respiratory distress syndrome are toxic to sheep pulmonary endothelial cells. Am J Resp Crit Care Med 2000; 162:288–94. [DOI] [PubMed] [Google Scholar]
- 13. Ordija C, Chiou TT, Yang Z, Deloid GM, de Oliveira Valdo M, et al. . Free actin impairs macrophage bacterial defenses via scavenger receptor MARCO interaction, with reversal by plasma gelsolin. Am J Physiol Lung Cell Mol Physiol 2017; 312: L1018-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem 2000; 275:14573–8. [DOI] [PubMed] [Google Scholar]
- 15. Osborn TM, Dahlgren C, Hartwig JH, Stossel TP. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am J Physiol Cell Physiol 2007; 292:C1323–30. [DOI] [PubMed] [Google Scholar]
- 16. Bucki R, Kulakowska A, Byfield F, et al. . Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am J Physiol Cell Physiol 2010; 299:C1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Chiou TT, Stossel TP, Kobzik L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol Lung Cell Mol Physiol 2015; 309:L11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bucki R, Georges PC, Espinassous Q, et al. . Inactivation of endotoxin by human plasma gelsolin. Biochemistry 2005; 44:9590–7. [DOI] [PubMed] [Google Scholar]
- 19. Bucki R, Byfield F, Kulakowska A, et al. . Extracellular gelsolin binds lipoteichoic acid and modulates cellular response to proinflammatory bacterial wall components. J Immunol 2008; 181:4936–44. [DOI] [PubMed] [Google Scholar]
- 20. Weiner DJ, Bucki R, Janmey PA. The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol 2003; 28:738–45. [DOI] [PubMed] [Google Scholar]
- 21. Bucki R, Durnaś B, Wątek M, et al. . Targeting polyelectrolyte networks in purulent body fluids to modulate bactericidal properties of some antibiotics. Infect Drug Resist 2018; 11: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mounzer KC, Moncure M, Smith YR, DiNubile MJ. Relationship of admission gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med 1999; 160:1673–81. [DOI] [PubMed] [Google Scholar]
- 23. Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg 2006; 243:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. . Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung 2002; 180: 91–104. [DOI] [PubMed] [Google Scholar]
- 25. Lind SE, Smith DB, Janmey PA, Stossel TP. Depression of gelsolin levels and detection of gelsolin-actin complexes in plasma of patients with acute lung injury. Am Rev Respir Dis 1988; 138:429–34. [DOI] [PubMed] [Google Scholar]
- 26. Smith DB, Janmey PA, Lind SE. Circulating actin-gelsolin complexes following oleic acid-induced lung injury. Am J Pathol 1988; 130:261–7. [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Cui F, Cheng Y, et al. . Gelsolin: role of a functional protein in mitigating radiation injury. Cell Biochem Biophys 2015; 71:389–96. [DOI] [PubMed] [Google Scholar]
- 28. Lee PS, Waxman AB, Cotich KL, Chung SW, Perrella MA, Stossel TP. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit Care Med 2007; 35:849–55. [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Cheng B, Chen Q, et al. . Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit Care 2008; 12:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang LF, Yao YM, Li JF, et al. . Reduction of plasma gelsolin levels correlates with development of multiple organ dysfunction syndrome and fatal outcome in burn patients. PLoS One 2011; 6:e25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu JF, Liu WG, Dong XQ, Yang SB, Fan J. Change in plasma gelsolin level after traumatic brain injury. J Trauma Acute Care Surg 2012; 72:491–6. [DOI] [PubMed] [Google Scholar]
- 32. Chou SH, Lee PS, Konigsberg RG, et al. . Plasma-type gelsolin is decreased in human blood and cerebrospinal fluid after subarachnoid hemorrhage. Stroke 2011; 42:3624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. . Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J Investig Med 2002; 50: 54–60. [DOI] [PubMed] [Google Scholar]
- 34. Kaneva MK, Greco KV, Headland SE, et al. . Identification of novel chondroprotective mediators in resolving inflammatory exudates. J Immunol 2017;198: 2876–85. [DOI] [PubMed] [Google Scholar]
- 35. Piktel E, Levental I, Durnas B, Janmey PA, Bucki R. Plasma gelsolin: indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int J Mol Sci 2018; 19: 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rothenbach PA, Dahl B, Schwartz J, et al. . Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol 2004; 96:25–31. [DOI] [PubMed] [Google Scholar]
- 37. Yang Z, Levinson S, Stossel T, DiNubile MJ, Kobzik L. Delayed therapy with plasma gelsolin improves survival in murine pneumococcal pneumonia [Abstract]. Open Forum Infect Dis 2017; 4 (Suppl 1): S474–5. [Google Scholar]
- 38. Fine MJ, Auble TE, Yealy DM, et al. . A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 39. Charles PGP, Wolfe R, Whitby M, et al. . SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47: 375–84. [DOI] [PubMed] [Google Scholar]
- 40. Self WH, Grijalva CG, Williams DJ, et al. . Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest 2016; 150:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression and survival analysis. New York: Springer; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.