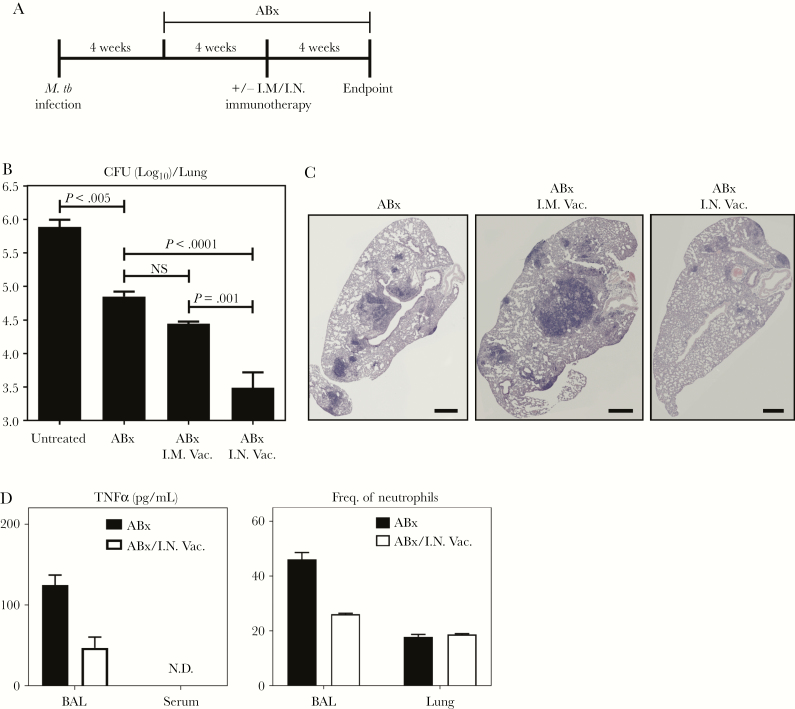
Figure 1.
AdCh68Ag85A respiratory mucosal immunotherapy improves tuberculosis (TB) disease control during antibiotic therapy. (A) Experimental schema. At 4 weeks post-Mycobacterium tuberculosis (M.tb) infection, mice were started on an oral antibiotic (antibiotics alone [ABx]) therapy of rifampicin, isoniazid, and pyrazinamide. A group of these mice was treated either intramuscularly (ABx I.M Vac.) or intranasally (ABx I.N. Vac.) with AdCh68Ag85A at 4 weeks after the initiation of antibiotic therapy. All mice were sacrificed 12 weeks postinfection for assessment of TB disease indices. A set of M.tb-infected animals were left untreated as controls (untreated). (B) Bar graph comparing bacterial burden assessed by colony-forming unit (CFU) assay in the lungs of 4 groups of mice. (C) Representative micrographs of lung sections stained with hematoxylin and eosin, comparing the extent of lung inflammation and granulomatous lesions. Scale bar indicates 500 µm. (D) Bar graphs showing levels of tumor necrosis factor α (TNFα) protein in the bronchoalveolar lavage (BAL) fluid and in sera, and frequencies of neutrophils (CD11b+Ly6G+) in the BAL and lung at 72 hours post-I.N. immunotherapy. Data are expressed as the mean ± standard error of the mean of 6–10 mice/group, representative of 3 independent experiments.