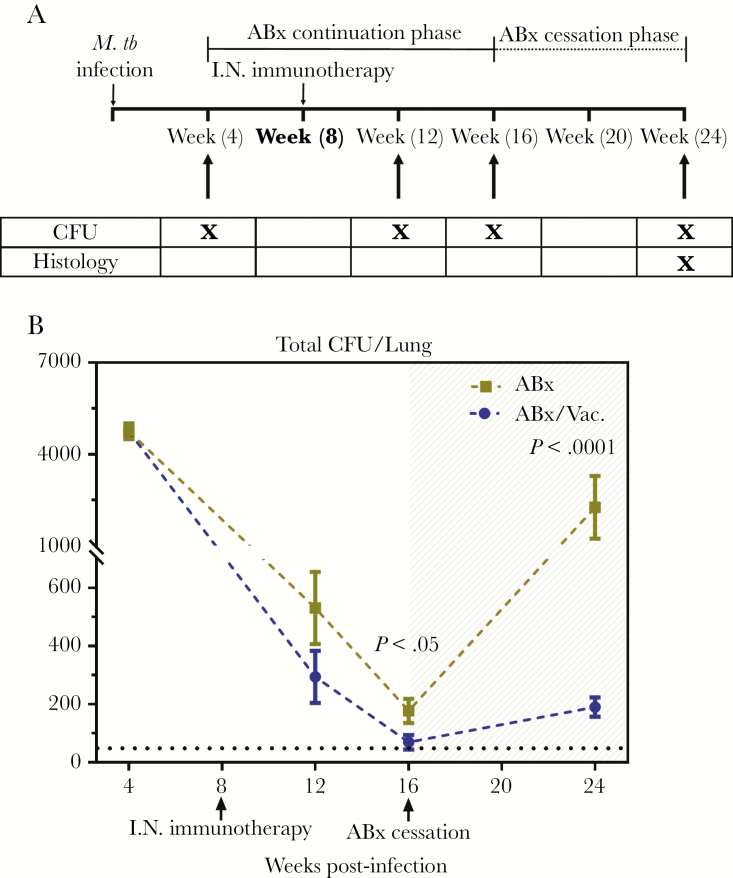
Figure 4.
Efficacy of AdCh68Ag85A respiratory mucosal immunotherapy in a low infection dose model. (A) Experimental schema. Mice were infected and treated as described in Figure 3A (schema) but with a low dose of Mycobacterium tuberculosis (M.tb) infection (100 colony-forming units [CFU]), and antibiotic therapy ceased at week 16 postinfection. At specified time points, tuberculosis disease indices were assessed. (B) Line graph showing kinetic changes in bacterial burden in the lung. Unshaded area indicates antibiotic continuation phase and shaded area indicates antibiotic cessation phase. Dotted horizontal line represents the limit of detection (50 CFU). Data are expressed as the mean ± standard error of the mean of 6–12 mice/group, representative of 1 to 3 independent experiments (depending on the time point). ABx, antibiotics alone; I.N., intranasal.