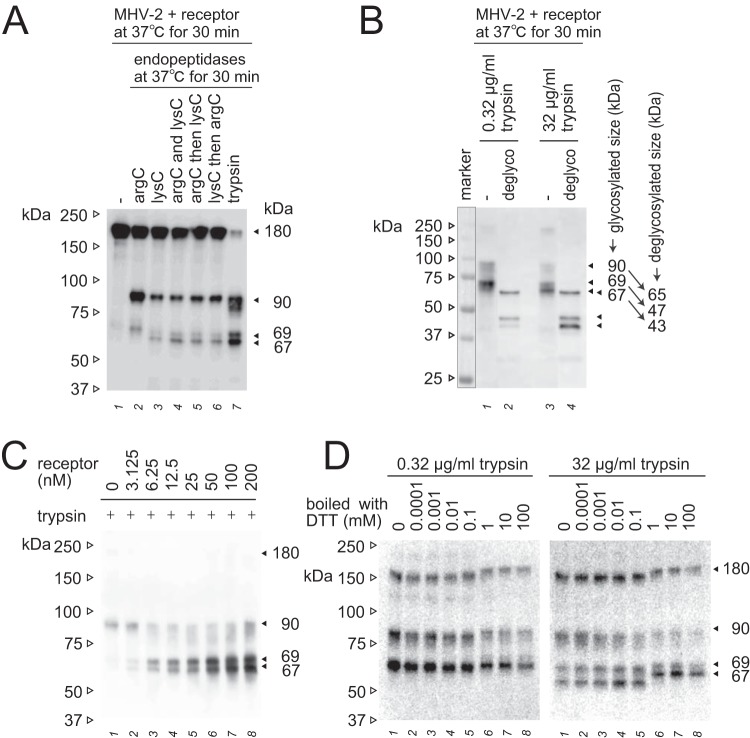
FIG 3.
Additional characterization of the 67- and 69-kDa subunits. (A) Specific cleavage of the postS2′ site at arginine or lysine residues. Endopeptidase arg-C (20 μg/ml) and/or lys-C (20 μg/ml) were employed instead of trypsin to induce S protein conformational changes. (B) Deglycosylation of S2 subunits. S protein activated by receptor and trypsin was deglycosylated using a deglycosylation enzyme mix. Nonrelevant lanes on the same blot were sliced out in Adobe Photoshop to align the lanes shown. (C) Receptor concentration dependence. MHV-2 was treated with serially diluted soluble receptor and then with trypsin (10 μg/ml). (D) Effect of redox potential. The 67- and 69-kDa subunits induced by the treatment of receptor and trypsin (0.32 and 32 μg/ml) were boiled in sample buffer containing the indicated concentration of DTT. For all panels, after SDS-PAGE, Western blot analysis was carried out using MAb-10G antibody.