Fig. 1. Optimization of the assembly between cyanine dyes and albumin produced an efficient NIR-II complex.
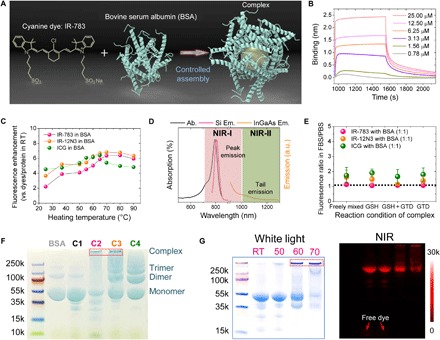
(A) Simplified design for the formation of the fluorophore@BSA complex. (B) Kinetic binding assay of IR-783 to albumin was measured by biolayer interferometry with Kd value ~1 nM. (C) Brightness enhancement after heating for 10 min of dyes premixed with BSA (100 μM). (D) Absorption and NIR-I and NIR-II emission of IR-783 in 100 μM BSA solution. Ab., absorption spectra; Si Em., emission spectra recorded on silicon camera; InGaAs Em., emission spectra recorded on InGaAs camera. (E) Fluorescence enhancement of the fluorophore (IR-783, IR-12N3, and ICG) @BSA complex (1:1 ratio) at 60°C. The IR-783:BSA complex is the most stable, with a fluorescence ratio closest to 1. GTD, glutaraldehyde. (F) Electrophoresis analysis of IR-783@BSA complexes (C1 to C4 were synthesized with 10-min posttreatment at 60°C). C2 and C3 have a large population close to the top compared with C1 and C4. (G) Electrophoresis gel analysis of IR-783@BSA complexes [prepared from C2: glutathione (GSH) and heat] with 10 min posttreatment at different temperatures [room temperature (RT), 50°, 60°, and 70°C]. At higher temperatures, a higher–molecular weight complex was observed.
