Fig. 2. The possible interaction mechanism between IR-783 and albumin is critical to guide efficient nanocomplex synthesis and fluorescence enhancement.
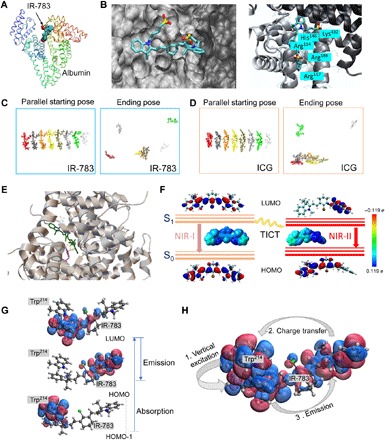
(A) Docking modeling for the IR-783@albumin complex. (B) Details of binding residues and active pocket between IR-783 and albumin. (C and D) The intermolecular stacking of IR-783 and ICG. ICG showed relatively higher tendency to be self-assembling compared with IR-783. (E) Docking modeling for IR-783 and albumin with partial disulfide bond cleavage, affording strong interaction between IR-783 and the Trp residue of albumin. (F) Schematic illustration of the transformation of TICT induced NIR-I and NIR-II states of IR-783, including the HOMOs and LUMOs and the ESP maps that are plotted using the color range from red [−0.119 electron (e), negative] to blue (0.119 electron, positive). S0, ground state; S1, excited state. (G) The LUMO (top), HOMO (middle), and HOMO-1 (bottom) are the three major molecular orbitals involved in the absorption (HOMO-1 → LUMO) and emission (LUMO→HOMO) processes. HOMO-1 lies on the indole ring of Trp214, while LUMO and HOMO are lying on the closer and further side of IR-783 with respect to Trp214. (H) The absorption and emission form a cycle with three steps: (1) vertical excitation (HOMO-1 → LUMO), (2) charge transfer from IR-783 to Trp214 (HOMO → HOMO-1), and (3) emission (LUMO → HOMO).
