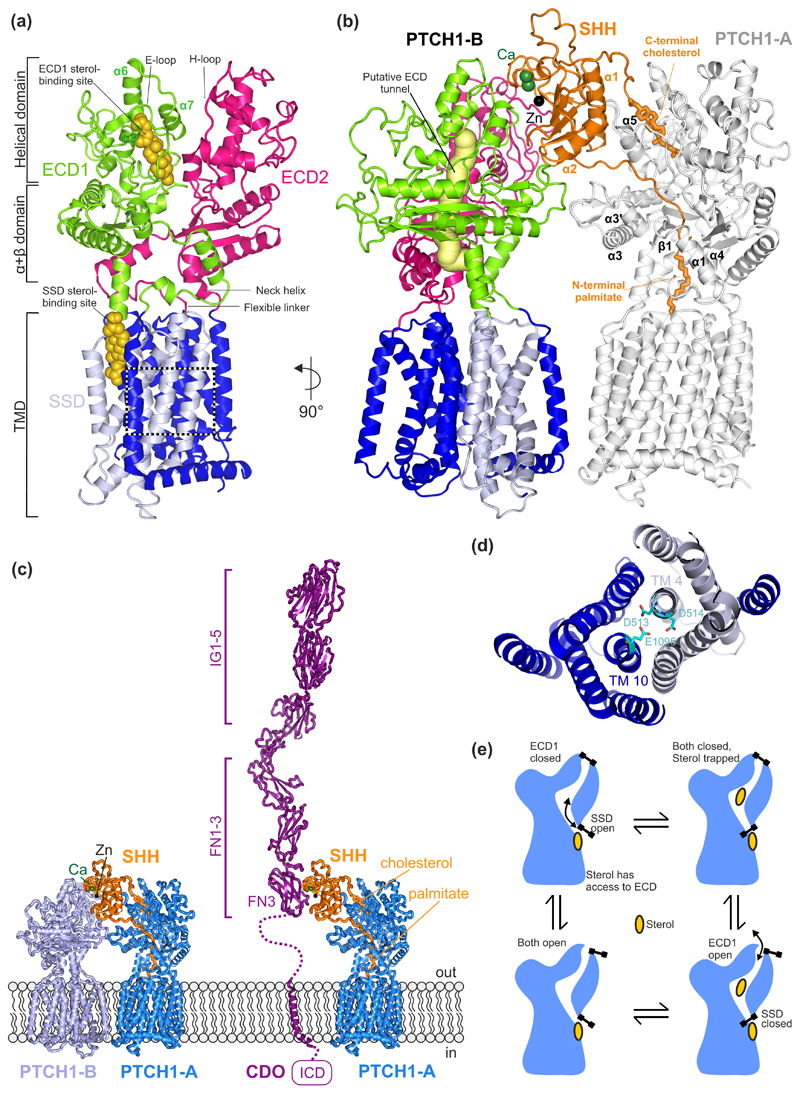
Figure 2. Structures of PTCH1 and their functional implications.
(a) The overall structural arrangement of monomeric PTCH1 (PDB 6DMB [19]). Each ECD is connected to the TMD through a flexible linker at the N-terminus and a neck helix at the C-terminus. Each ECD contains an α+β domain, located immediately above the TM, followed by a helical domain consisting of α-helices and lengthy loops. The E loop on ECD1 and the H loop on ECD2 contain residues important for SHH binding. Two sterol-like molecules have been identified in the structures, one located in ECD1 and another one in the SSD at the level of the outer leaflet of the membrane. (b) Cryo-EM structure of the 2:1 PTCH1:SHH complex (PDB 6E1H [21]) containing two PTCH1 molecules labelled PTCH1-A and PTCH1-B. A putative tunnel (yellow) through PTCH1 is proposed to be the conduit for sterol flow from the ECD1 to the SSD. The N-terminal palmitoyl appendage of SHH occludes this tunnel and the C-terminal cholesterol appendage occludes the sterol binding site in ECD1, presumably blocking transport. These lipidic appendages, together with α1 and α2 of SHH, form the major SHH-PTCH1-A binding interface. SHH interacts with PTCH1-B through an interface organized by the Ca2+-binding site in SHH. (c) Structural model of the CDO:SHH:PTCH1 co-receptor complex. CDO occupies a similar position as PTCH-B. The model of CDO:SHH was generated using the HHPRED [63] webserver as well as the crystal strcture structure of the CDO-FN3:SHH complex (PDB ID. 3D1M [25]). Ig: immunoglobulin-like domain, FN: fibronectin type III-like domain, ICD: intracellular domain. (d) Close-up of a charged triad, formed by PTCH1 residues D513, D514 (TM4) and E1095 (TM10) that may facilitate cation flow down a concentration gradient to power sterol transport. Mutations in this region have been shown to abolish the function of PTCH1. (e) Ordered conformational cycling of PTCH1, driven by cation flow, may enforce the directionality of sterol movement through PTCH1. An “alternating gate” mechanism is proposed where conformational changes (stylized as gates) either lead to the opening of the gate in the membrane, near the SSD site, or the mouth of the ECD1. Sterol flow could be in either direction, from the SSD site to the ECD1 (as shown) or vice versa.