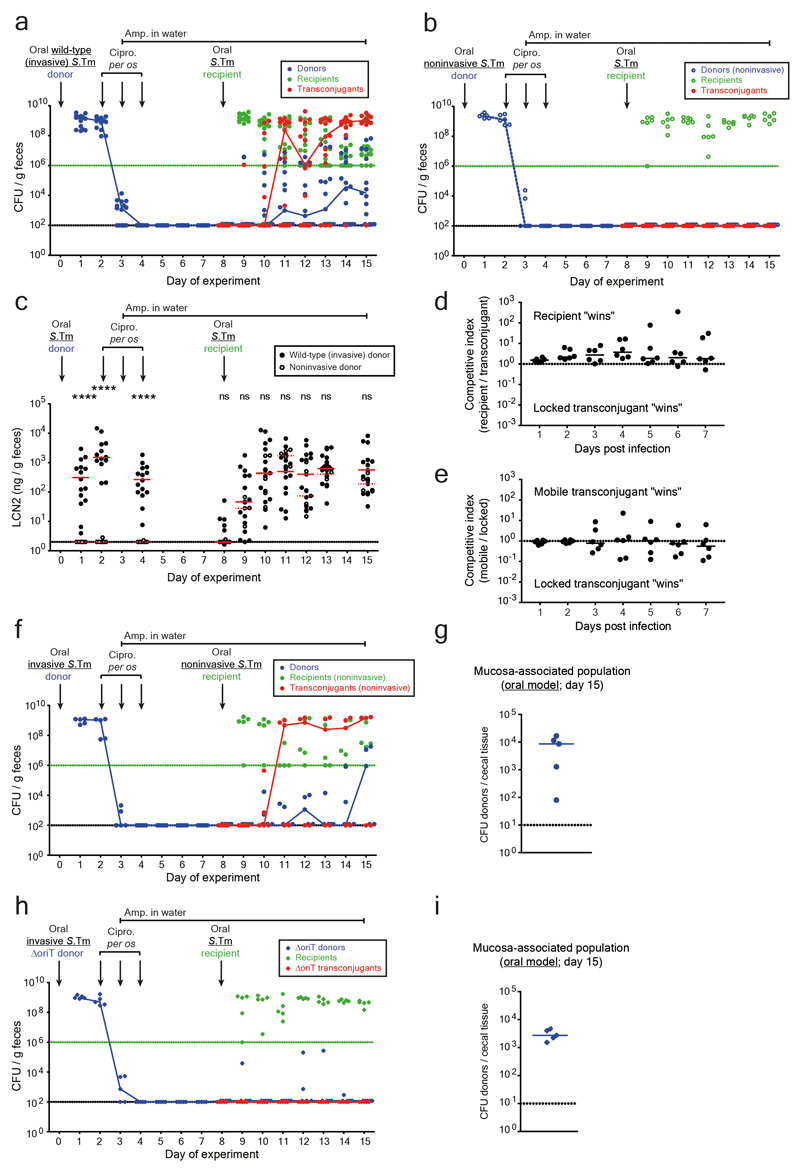
Extended data Fig. 3. Controls for conjugation after antibiotic treatment in the oral infection model.
a-c) Fecal bacterial population sizes and inflammatory status of mice in Figure 1c-d (comparison of invasive vs. noninvasive donors in the oral model). Fecal loads of donors (blue; SmR, CmR), recipients (green; KanR), and transconjugants (red; CmR, KanR) were determined by selective plating on MacConkey agar. Black dotted line indicates detection limits for donors and transconjugants. Green dotted line indicates detection limit for recipients (the detection limit is higher for recipients once transconjugants reach a density of >108 CFU/g feces. Before this happens, recipients can be found below the detection limit and then the black dotted line should be considered as the detection limit). Blue lines connect medians of donor populations; red lines connect medians of transconjugant populations. a) Mice infected with invasive S.Tm donors (solid circles; n=15 singly housed mice from five independent experiments). b) Mice infected with noninvasive S.Tm donors (open circles; n=6 singly housed mice from two independent experiments). c) Inflammatory status was determined by lipocalin-2 ELISA. Statistics are performed using a two-tailed Mann-Whitney U test p>0.05 (ns), p<0.0001 (****) comparing mice infected with an invasive donor (solid black circles; n=15 singly housed mice from five independent experiments) to mice infected with a noninvasive donor (open black circles; n=6 singly housed mice from two independent experiments) at each time point. Medians are shown (solid red line for invasive donors; dotted red line for noninvasive donors). Dotted line indicates detection limit. d-e) Carrying P2cat does not lead to a measurable fitness cost or benefit. d) A "locked" transconjugant (14028S P2aphT ΔoriT; conjugation is blocked by removing the origin of transfer; KanR, AmpR) was competed against a recipient (14028S cat; CmR, AmpR). e) To ensure removing the origin of transfer did not affect fitness, the locked transconjugant was competed against a transconjugant with a normal P2cat plasmid (i.e., mobile transconjugant) (14028S P2cat; CmR, AmpR). In panel d-e, both strains were introduced at a 1:1 ratio (total inoculum size ~5×107 CFU per os) and feces were monitored daily by selective plating (n=6 singly housed mice from two independent experiments for both experiments). Competitive index is calculated by dividing the population size of one competitor by the other. Lines indicate medians. The dotted line indicates no competitive advantage for either strain. This is in line with published data 20. These data indicate that it is the plasmid-encoded conjugation efficiency (not its effects on host bacterial fitness) that drives the prodigal rise of transconjugants (Fig. 1c). f-g) Plasmid transfer in the oral model does not require an invasive recipient. Invasive donors (SL1344 P2cat; SmR, CmR) were orally infected into pretreated mice. After ciprofloxacin treatment, a noninvasive mutant of S.Tm 14028S was used as a recipient (14028Snoninv aphT (KanR, AmpR); n=5). f) Selective plating determined fecal loads of donors (blue; SmR, CmR), recipients (green; KanR), and transconjugants (red; CmR, KanR). Black dotted line indicates detection limit for donors and transconjugants. Green dotted line indicates detection limit for recipients (the detection limit is higher for recipients once transconjugants reach a density of >108 CFU/g feces. Before this happens, recipients can be found below the detection limit and then the black dotted line should be considered as the detection limit). Blue lines connect medians of donor populations; red lines connect medians of transconjugant populations. g) Donor populations enumerated after a gentamycin protection assay on cecal tissue of mice shown in panel f. Median indicated by solid line. Dotted line indicates detection limit. h-i) conjugation is required for plasmid transfer after antibiotic treatment. Mice were infected with invasive S.Tm lacking the origin of transfer in P2cat (SL1344 P2cat ΔoriT; SmR, CmR) as a donor, and 14028S aphT (KanR, AmpR) as a recipient after antibiotic treatment (n=5). h) Selective plating determined fecal loads of donors (blue; SmR, CmR), recipients (green; KanR), and transconjugants (red; CmR, KanR). Black dotted line indicates detection limit for donors and transconjugants. Green dotted line indicates detection limit for recipients (the detection limit is higher for recipients once transconjugants reach a density of >108 CFU/g feces. Before this happens, recipients can be found below the detection limit and then the black dotted line should be considered as the detection limit). Blue lines connect medians of donor populations; red lines connect medians of transconjugant populations. i) Donor populations enumerated after a gentamycin protection assay on cecal tissue of mice shown in panel h. Median indicated by solid line. Dotted line indicates detection limit.