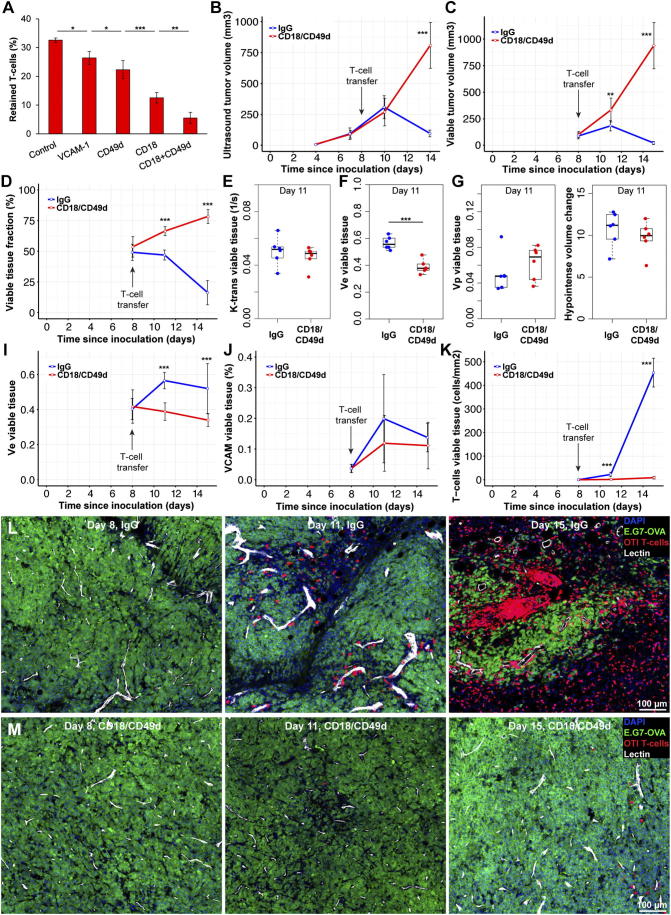
Figure 4.
Blocking T-cell binding to endothelial cells, prevented tumor rejection in an adoptive transfer model. (A) T-cell retention on stimulated endothelial cells experiencing 5 dyn/cm2 shear stress was measured using parallel plate flow chambers. Incubating endothelial cells with VCAM-1 antibodies prior to T-cell binding experiments reduced T-cell retention significantly (P < .05, n = 4). Incubation with CD49d or CD18 antibodies led to significant reductions in cell retention compared to VCAM-1 and CD49d respectively (P < .05, P < .001, n = 4). Combining CD18 and CD49d reduced T-cell retention further compared to CD18 alone (P < .01, n = 4) (B) Ten million activated CD8+ OTI (OVA specific) T-cells were adoptively transferred into each NSG mouse with an E.G7-OVA tumor growing on their right hind leg. Ultrasound-based tumor volume measurements showed no differences between IgG and CD18/CD49d groups 2 days after adoptive T-cell transfer. However, 4 days later tumor volumes were significantly different (P < .001, n = 6/group). (C) MRI based multispectral tissue classification found significant differences in viable tumor volumes 3 days (P < .01, n = 6/group) after T-cell transfer which did increased further by day seven (P < .001). (D) Differences between IgG and CD18/CD49d groups were more pronounced when viable tumor tissue was normalized to tumor volume. (E) Tumor perfusion and permeability (k-trans) was not significantly different between IgG and CD18/CD49d groups 3 days after adoptive T-cell transfer. (F) The extracellular extravascular volume fraction of viable tumor tissue was significantly different between IgG and CD18/CD49 groups (P < .001, n = 6/group) 3 days after T-cell transfer. (G,H) Plasma volume (vp) and hypointense volume changes were not different between groups 3 days after T-cell transfer in viable tumor tissue. (I) The extracellular extravascular volume fraction in viable tumor tissue increased significantly during T-cell mediated tumor rejection (IgG group) while exponentially growing tumors (CD18/CD49d group) showed a slight decrease in ve (n = 6/group, P < .001). (J,K) While histological assessment of vascular VCAM-1 density did not show significant differences between IgG and CD18/CD49d groups, T-cell density was significantly different 3 days after T-cell transfer (P < .001, n = 6/group). T-cell density in the IgG group increased >10-fold between day three and seven post T-cell transfer. (L,M) Representative confocal microscopy images showing T-cell accumulation around perfused vessels in the IgG group by day 11 (3 days after T-cell transfer) with few T-cells in tumors of mice treated with CD18/CD49d antibodies. Four days later, tumors of IgG treated animals contained only small areas of viable tumor cells surrounded and infiltrated by large amounts of T-cells. T-cell density in CD18/CD49d treated mice had increased marginally at day 15. DAPI: 4′,6-Diamidino-2-Phenylindole, E.G7-OVA: GFP expressing E.G7 tumor line, OTI T-cells: tdTomato expressing OTI (OVA specific) T-cells, Lectin: perfused vessel. Scale bars: 100 μm. Data shown as mean ± SD. Hair removal had been performed prior to ultrasound and MRI imaging on all tumors used for this figure.