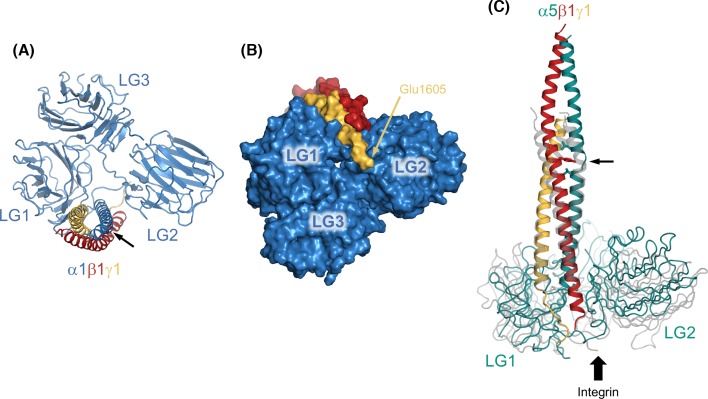
Figure 4. Crystal structures of heterotrimeric integrin-binding laminin fragments.
Integrin binding requires the C-terminal portion of the heterotrimeric coiled coil, together with domains LG1-3 of the laminin α chain [5]. (A) Structure of the laminin-111 mini-E8 fragment [35] viewed down the α-helical coiled coil, i.e., from N- to C-terminus. This view shows the ‘top’ surface of the LG1-3 triangle. The arrow indicates a kink in the α1 helix introduced by Pro2095. (B) Space-filling representation of the mini-E8 structure showing the ‘bottom’ surface of the LG1-3 triangle. The position of the critical integrin-binding E1605 residue (mouse γ1 chain) is indicated. The views in (A) and (B) are related by a 180° rotation about the horizontal axis. (C) Structure of the laminin-511 E8-like fragment [36] viewed from the side, superimposed onto the corresponding laminin-111 structure (in transparent gray). The arrow indicates an interruption to the regular heptad repeats in the α5 chain (see text). Integrin binding to the γ1 chain tail is indicated by the block arrow.