Abstract
Introduction
Polyglycolic acid (PGA) nerve conduits, an artificial biodegradable nerve regeneration-inducing tube currently used in clinical practice, are effective in regenerating peripheral nerves. Dedifferentiated fat (DFAT) cells differentiate into various cells including adipocytes, osteoblasts, chondrocytes, skeletal muscle cells, and myofibroblasts, when cultured in appropriate differentiation-inducing conditioned culture medium. This study made a hybrid artificial nerve conduit by filling a PGA conduit with DFAT cells, applied the conduit to a rat facial nerve defect model, and investigated the facial nerve regenerative ability of the conduit.
Methods
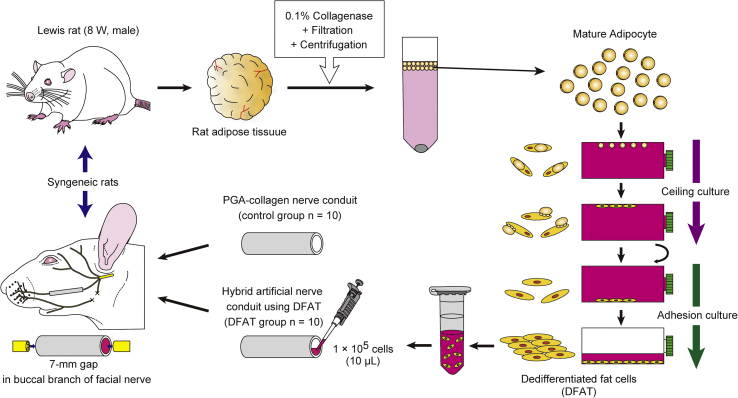
Under inhalational anesthesia, the buccal branch of the facial nerve in Lewis rats was exposed, and a 7-mm nerve defect was created. PGA nerve conduits were filled with DFAT cells, which were prepared from rat subcutaneous adipose tissue with type I collagen as a scaffold, and then grafted into the nerve defect sites in rats with a microscope (DFAT group) (n = 10). In other rats, PGA artificial nerve conduits alone were similarly grafted into the nerve defect sites (the control group) (n = 10). Reinnervation was confirmed at 13 weeks postoperatively by a retrograde tracer, followed by histological and physiological comparative studies.
Results
The mean number of myelinated fibers was significantly higher in DFAT group (1605 ± 806.23) than in the control group (543.6 ± 478.66). Myelin thickness was also significantly lager in DFAT group (0.57 ± 0.17 μm) than in the control group.
(0.46 ± 0.14 μm). Although no significant difference was found in the amplitude of compound muscle action potential (CMAP) between DFAT group (2.84 ± 2.47 mV) and the control group (0.88 ± 0.56 mV), whisker motion was lager in DFAT group (9.22° ± 0.65°) than in the control group (1.9° ± 0.84°).
Conclusions
DFAT cell-filled PGA conduits were found to promote nerve regeneration in an experimental rat facial nerve defect model.
Keywords: DFAT cells, Stem cell, Adipose tissue, Nerve regeneration
Abbreviations: ADSC, adipose-derived stem cell; DFAT, dedifferentiated fat; PGA, polyglycolic acid; PO, propylene oxide; SVF, stromal vascular fraction; VEGF, vascular endothelial growth factor
Highlights
-
•
PGA artificial conduits containing DFAT cells were made in this study.
-
•
The facial nerve regenerative ability of conduits was evaluated in a rat model.
-
•
Reinnervation was confirmed at 13 weeks postoperatively.
-
•
The nerve regeneration promoting effect of DFAT cells was found in the model.
1. Introduction
Autologous nerve transplantation with the sural nerve is a conventional treatment for facial nerve damage caused by trauma or malignant tumor resection. However, complications such as sensory paralysis and scarring in the donor area are unavoidable by this procedure [1]. For solving these drawbacks, artificial nerve regeneration-inducing conduits made from bioresorbable materials are developed, and many reports on the postoperative evaluation of peripheral nerve reconstruction with these conduits are found [2], [3]. However, the regenerating abilities of these conduits are found to be only approximately 50%–60% compared to conventional autologous nerve transplantation [4]. For improving the regenerative abilities of these artificial nerve regeneration-inducing conduits, various techniques such as filling the conduits with adipose-derived stem cells (ADSC) or stromal vascular fraction (SVF) are tested and reported [2], [3], [5], [6], [7], [8]. Dedifferentiated fat (DFAT) cells cultured from mature adipocytes recently attract attention in the field of regenerative medicine. Since mature adipocytes, which account for approximately 20%–30% of all adipocytes, are loosely bound each other, the cell can be readily isolated from adipose tissue by collagenase treatment. Characteristically, floating in liquid medium, isolated adipocytes can be readily cultured and propagated by ceiling culture, which allows cells to be cultured on the ceiling surface of medium-filled flask. Cells showing a fibroblast-like morphology are found during adipocyte ceiling culture, and Yagi et al. establish a mouse precursor adipocyte-cell line from these fibroblast-like cells that can differentiate into mature adipocytes, named DFAT [9]. DFAT cells can transdifferentiate not only into cells, which are identified to those derived from the paraxial mesoderm, but also into vascular endothelial cells, vascular smooth muscle cells, and myocardial cells derived from the splanchnic mesoderm [10]. Furthermore, the transplantation of DFAT cells into injured areas is found to result in a wide range of tissue repair and beneficial effects; improved blood flow in a lower-limb ischemia model, improved heart function in a myocardial infarction model, improved urination in a spinal cord injury model [11], improved kidney function effect in a chronic kidney disorder model [12], improved urination in a urethral sphincter disorder model [13], increased bone density in an osteoporosis model [14], and the promotion of skin regeneration after artificial dermal transplantation in a full-thickness skin defect model [15]. Because DFAT cells are prepared from the mature adipocyte fraction during the fractionation of mature adipocytes, highly pure cells can be obtained without complicated selection procedures, and the volume of tissue sample required is approximately 1 g or less [16]. Since DFAT cells are prepared from adipocytes in fat tissues that are usually discarded as medical wastes during surgery, DFAT cells preparation procedure has a great potential in clinical applications because of its noninvasiveness to donor areas during treatment. The authors previously reported that artificial nerve regeneration-inducing conduits made from silicone tubes filled with type I collagen and DFAT cells promote nerve regeneration in a rat facial nerve defective model [17]. For aiming the clinical application of the experimental system, this study created a hybrid artificial nerve conduit by filling clinically used collagen-coated polyglycolic acid (PGA) conduits with DFAT cells and investigated the facial-nerve regenerative potential of the hybrid conduits histologically and physiologically.
2. Materials and methods
All animal care and handling procedures were performed in accordance with the Principles of Laboratory Animal Care of Tokyo Women's Medical University Animal Experimentation Committee and Animal Research and Care Committee of the Nihon University School of Medicine. Eight-week-old male syngeneic Lewis rats (body weight: 200–250 g) (n = 20) were purchased from Charles River Laboratories (Yokohama, Japan).
2.1. Nerve conduits
The base material of the nerve conduits used in this study was a PGA tube with a length of 10 mm, an internal diameter of 1 mm, and an outer diameter of 1.5 mm (Nerbridge®) (Toyobo, Osaka, Japan), and the tubes were filled with collagen type I. PGA tube is commercially available and approved as a medical device for clinical practice by Japanese Government. The tube is confirmed to be biocompatible, and known to be dissolved and absorbed in the body for approximately 3 months. Collagen used in the tube was newly developed medical-grade collagen (NMP Collagen PS) (Nippon Meat Packers, Osaka) and applied to the outside and inside of the tube, and the collagen application allowed the blood vessels to penetrate into the tube easily and gave a suitable environment for the regeneration and guidance of peripheral nerves. PGA-collagen tubes are mainly used in Japan for repairing the peripheral sensory nerves [18].
2.2. Preparation of DFAT cells
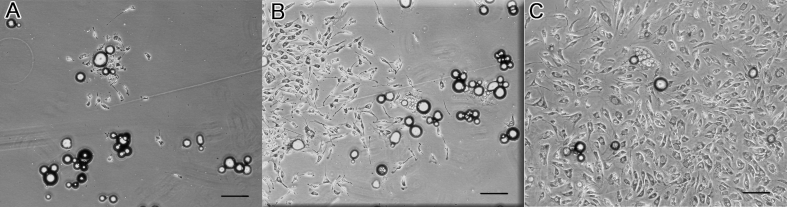
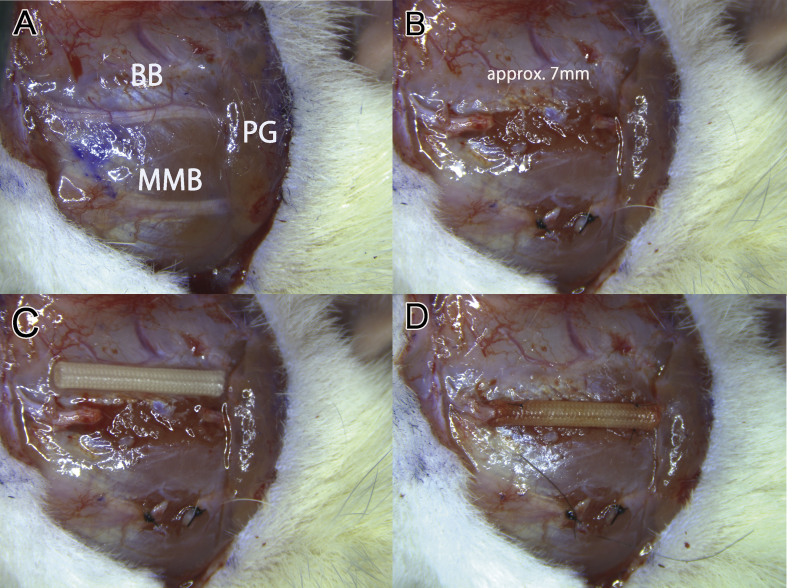
The preparation of DFAT cells from adipose tissue is performed as described previously [19]. Briefly, approximately 1 g isolated subcutaneous adipose tissue was minced and digested with 0.1% collagenase solution (collagenase type I) (Koken, Tokyo) at 37 °C for 1 h with gentle agitation. After filtration and centrifugation, the top layer containing floating unilocular adipocytes, which have a lower specific gravity than water, was collected. The adipocytes (5 × 104) were washed with phosphate-buffered saline (PBS), placed in a 25-cm2 culture flask filled completely with Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS) (JRH Bioscience, Lenexa, KS), and were incubated for 7 days (Fig. 1 B). Previously, the authors reproducibly isolated 4–6 × 106 adipocytes from approximately 1 g of subcutaneous adipose tissue and found that only 5 × 104 adipocytes are suitable for obtaining a sufficient number of DFAT cells (3 × 106 at primary culture). The adipocytes floating at the surface and adhering to the inner surface of the ceiling of the flask before differentiating to fibroblast-like DFAT cells were observe (Fig. 1A–C). The medium was then removed, and the flask was inverted for allowing the cells to stay on the bottom. The medium was exchanged every 3 days until the cells reached confluence. DFAT (1 × 105) with 10 μL type I collagen solution (Cellmatrix) (Nitta Gelatin, Osaka) was infused into a PGA-collagen nerve conduit with a micropipette (Fig. 2).
Fig. 1.
Microscopic images of dedifferentiated fat (DFAT) cells during culture. (A) At 4 days after the start of ceiling culture, cells maintained the morphology of mature adipocytes. (B) At 7 days, fibroblast-like cells arose from the mature adipocytes. (C) At 11 days, colony formation was observed around the mature adipocytes. The bars indicate 250 μm.
Fig. 2.
Surgical procedures for making in a facial nerve defect rat model and for transplanting nerve conduits. (A) Preauricular incision with a marginal mandibular extension on the left side of the head for exposing the buccal branch of the facial nerve and the parotid gland. BB indicates buccal branch; MMB, marginal mandibular branch of the facial nerve; PG, parotid grand. (B) A 7-mm defect was made in the buccal branch. Photograph C and D show that polyglycolic acid (PGA) conduits loaded with 10 μL of type I collagen gel with or without DFAT cells was transplanted into the nerve defect and fixed with a two-point mattress suture, respectively. The bars indicate 5 mm.
2.3. Surgical procedure and experimental design
Rats (n = 20) were anesthetized with 4% isoflurane through a nasal mask attached to a Univentor 400 Anesthesia Unit (Narcobit-E) (Natsume Seisakusho, Tokyo) (Fig. 3) [20]. A preauricular incision with a marginal mandibular extension was made on the left side of the face for exposing the buccal and marginal mandibular branches of the facial nerve and parotid gland (Fig. 2A). The marginal mandibular branch was transected with microsurgical scissors (MB-51-10) (Natsume Seisakusho) and ligated with 7-0 nylon sutures (Ethilon®, Ethicon) (Johnson & Johnson, New Brunswick, NJ). A 7-mm defect was made in the buccal branch (Fig. 2B) because of rat face's physical limitation [21]. PGA-collagen nerve conduits were flushed with physiological saline for 30 min before grafting. The defects were individually bridged with PGA-collagen nerve conduits alone (the control group) or hybrid PGA-collagen nerve conduits filled with type I collagen and DFAT (DFAT group) (Fig. 2C). PGA conduits were secured in place by the two-point mattress suture method reported previously (Fig. 2D) [22]. A 1-mm nerve stump was then inserted into the conduit by pulling both sutures. Surgery was performed with a microscope (M60) (Leica Microsystems, Wetzlar, Germany) (Fig. 3). At 13 weeks after implantation, the conduit was carefully excised with the nerve stump on the graft bed. A 13-week period yielded sufficient neural regeneration, allowing the physiological functions of regenerated nerve in conduit to be evaluated by compound muscle action potential (CMAP) measurement and a whisker motion detection camera.
Fig. 3.
Schematic overview of the experimental procedures.
2.4. CMAP recordings of the vibrissal muscles
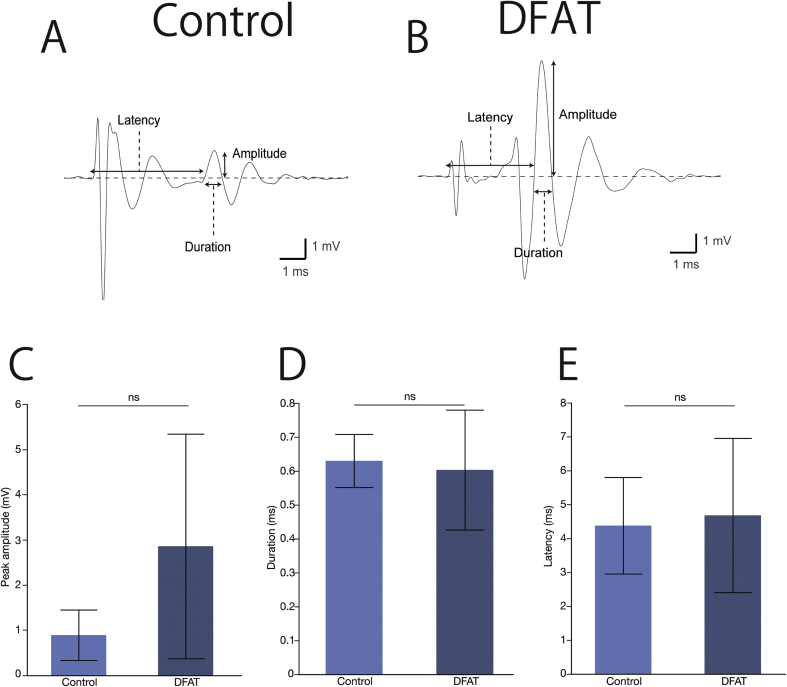
For evaluating nerve regeneration physiologically, CMAP was performed as previously reported [23]. Obtained data were analyzed off-line by Igor Pro software (WaveMetrics, Lake Oswego, OR). Rats were anesthetized with 4% isoflurane at 13 weeks after transplant, and the heads were fixed by a brain stereotaxic apparatus (SR-6N) (Narishige, Tokyo). Stainless-steel microelectrodes with an impedance of 9–12 MΩ at 1 kHz (UESMGCSELNNM-type) (FHC, Bowdoin, ME) were inserted into the vibrissal muscles of rows C and D to record CMAP by stimulating the regenerated buccal branch of the facial nerve. Standard electrodes (TN204-089B) (Unique Medical, Tokyo) were placed caudally on the skull. After the regenerated buccal branch was exposed, a tandem hook-shaped stimulation electrode (IMC-220224) (InterMedical, Nagoya) was attached to the regenerated nerves in the both groups. The nerves were stimulated with supramaximal stimulation pulses (∼2 mA, 100-μsec monopolar pulses) at a frequency of 0.2 Hz with an isolator (SS-202J) (Nihon Kohden, Tokyo). Recorded signals were processed with a multichannel amplifier (MEG-6100) (Nihon Kohden) at 15 to 10,000 Hz and then digitized at 40 kHz with PowerLab4/30 and LabChart7 systems (ADInstruments, Dunedin, New Zealand). An upward trace indicated a negative deflection (depolarization), and 10 consecutive traces were averaged. CMAP amplitude was measured as the difference between maximum and baseline voltages. Duration was calculated as the time between the two points where the baseline was crossed by the rising and declining CMAP curves. Latency was estimated as the time between the stimulus artifact and the point where the baseline was crossed by the rising CMAP curve (Fig. 4A and B).
Fig. 4.
Compound muscle action potential (CMAP) analysis. CMAP wave patterns recorded from the whisker pad of rat after supramaximal stimulation in the control (A) and DFAT (B) groups. (C) There were no significant differences between the control and DFAT groups in amplitude (0.88 ± 0.56 mV vs. 2.8 ± 2.5 mV), (D) duration (0.63 ± 0.078 mV vs. 0.60 ± 0.18 mV), and (E) latency (4.38 ± 1.42 ms vs. 4.68 ± 2.27 ms). The bars above and under the columns show the standard deviations (SDs). The abbreviation “n.s.” indicates non-significance.
2.5. Whisker motion detection
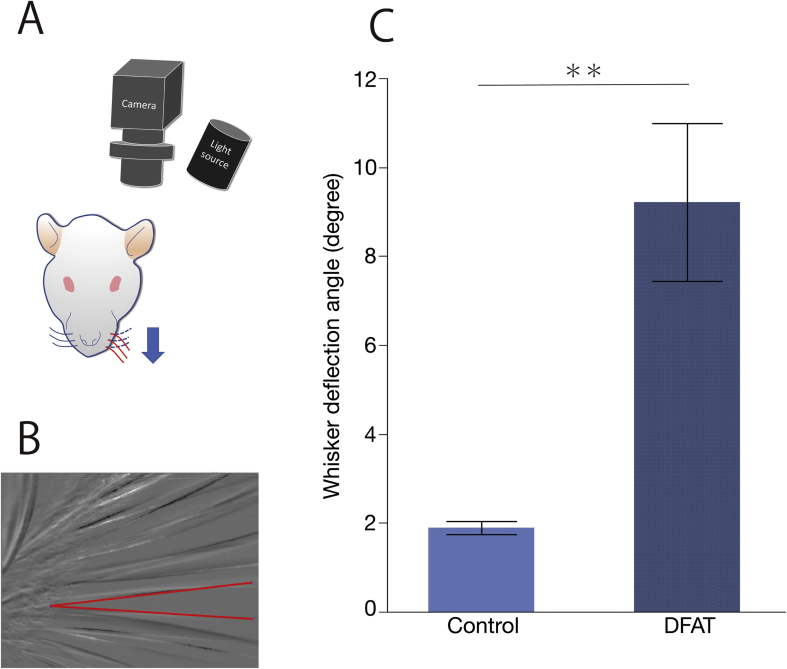
Along with CMAP recordings, whisker motion was recorded with an industrial camera. Recordings were made at 500 Hz with an Ultra-Compact USB3 Vision industrial camera (XIMEA) (Munster, Germany) and a light source with a central wavelength of 780 nm (LIU780A) (THORLABS, Newton, NJ). Whisker motion was measured by stimulating the buccal branch during CMAP recordings described above. MATLAB (The MathWorks, Natick, MA) was used to create a program for analyzing the recorded video data. Whisker motion data of the control group ware compared to those of DFAT groups by obtaining difference in the angles between the whiskers stages of whisker pad at the relaxation and contraction (Fig. 5A and B).
Fig. 5.
Schematic illustration of the experimental setup of whisker motion measurement and the result. (A) Whisker motion of the control group was compared with that of dedifferentiated fat (DFAT) cell groups by obtaining differences in the angles between the whiskers at stages of whisker pad relaxation and contraction. (B) In the expanded photograph of the whiskers, the red lines indicate the angles of the whiskers of relaxation and contraction whisker pads. (C) Whisker motion was greater in DFAT group (9.22° ± 0.65°) than in the control group (1.90° ± 0.84°). The bars above and under the columns show the standard deviations (SDs). Two asterisk (**) indicate a probability of less than 0.01 (p < 0.01).
2.6. Retrograde labeling of facial motoneurons through the regenerated nerve
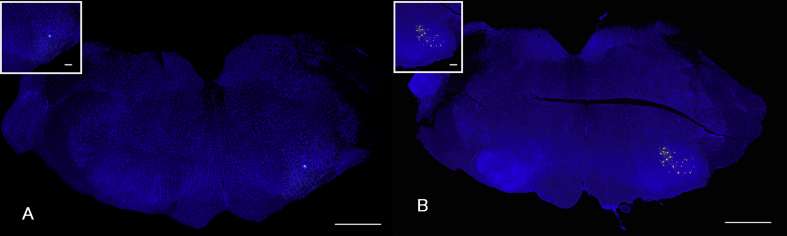
Rats were anesthetized with 4% isoflurane at 11 weeks after transplantation and injected with retrograde fluorescence tracer DiI (D-28) (Invitrogen) into the left whisker pad with a 25-μL Hamilton syringe. At 2 weeks after injection, rats were deeply anesthetized with 4% isoflurane and perfused transcardially with fixative solution containing 4% paraformaldehyde and 0.2% picric acid in 0.1 mol/L phosphate buffer. The brain was then removed and postfixed with the same fixative solution overnight. Coronal 50-μm sections of the brainstem were prepared with a vibratome (VT1000) (Leica Microsystems, Buffalo Grove, IL), washed with 0.1 mol/L PBS, mounted on gelatin-coated glass slides, and cover-slipped with aqueous mounting medium. Fluorescent signal of DiI was observed with a cooled CCD camera (Quantum Scientific Imaging, Poplarville, MS).
2.7. Histological analysis of myelinated fibers
At 13 weeks after transplantation, the center of the regenerated buccal branch of the rat facial nerve was resected and stained with toluidine blue for counting the number of myelinated fibers. Actually, the specimens were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 mol/L cacodylate buffer at pH 7.4 at 4 °C overnight, then washed 3 times with 0.1 mol/L cacodylate buffer for 30 min, and postfixed with 2% osmium tetroxide in 0.1 mol/L cacodylate buffer for 3 h at 4 °C. Graded ethanol solutions (50, 70, 90, and 100%) were used for dehydration with the following schedule: 50% and 70% for 30 min individually at 4 °C, 90% for 30 min at room temperature, and 4 repetitions of 100% for 30 min at room temperature. Dehydration was then continued overnight with 100% ethanol at room temperature. Infiltration of the specimens was performed by washing in propylene oxide (PO) 2 times for 30 min and then incubating in a 70:30-mixture of PO and epoxy resin (Quetol-812) (Nisshin EM, Tokyo) for 1 h. The tube containing the specimen was left uncovered for allowing PO to volatilize overnight, resulting in concentrating the resin. Specimens were transferred into a fresh 100% resin solution, which was allowed to polymerize for 48 h at 60 °C. Ultra-thin 1.5-μm sections were prepared from the polymerized resin with a glass knife and an ultramicrotome (Ultracut UCT) (Leica, Vienna, Austria), and stained with 0.5% toluidine blue.
2.8. Transmission electron microscopy of the regenerated nerves
For accurately observing the morphology of the regenerated nerve axons and myelin, the resin-embedded specimen was cut into 70-nm sections, which were mounted on grids (EM fine-grid F-200) (Nisshin EM). Sections were then stained with 2% uranyl acetate and lead citrate solution (Sigma–Aldrich, St. Louis, MO.) and examined with a JEM1200EX transmission electron microscope (JEOL, Tokyo) with an accelerating voltage of 80 kV. Fiber diameter, axon diameter, and myelin thickness of randomly selected axons from five transmission electron microscopic images at a magnification of 3550 were measured by Photoshop CS2 (Adobe Systems, San Jose, CA).
2.9. Statistical analysis
Results were expressed as the mean ± standard deviation, and a p-value less than 0.05 was considered significant. The number of myelinated fibers, fiber diameter, axon diameter, myelin thickness, and g ratio (axon diameter/fiber diameter) [24], CMAP amplitude, duration, and latency, and Whisker motion in both groups were analyzed by Student's t-test with JMP® software, version 13 (SAS Institute Inc., Cary, NC).
3. Results
3.1. DFAT cell pluripotency induced by differentiation medium
At passage 5, DFAT cells maintained the squamous morphology of fibroblasts and exhibited an auto-regenerative ability (Fig. 1).
3.2. Physiological properties of regenerated facial nerves regeneration at 13 weeks after transplantation
CMAP wave pattern of DFAT group was similar to that of the control group (Fig. 5A and B). There were no significant differences between the control and DFAT groups in amplitude (0.88 ± 0.56 mV vs. 2.8 ± 2.5 mV) (Fig. 4C), duration (0.63 ± 0.078 mV vs. 0.60 ± 0.18 mV) (Fig. 4D), and latency (4.38 ± 1.42 ms vs. 4.68 ± 2.27 ms) (Fig. 4E).Whisker motion in DFAT group (9.22° ± 0.65°) was greater than that in the control group (1.90° ± 0.84°)
(Fig. 5B and C). Fluorescent retrograde neuronal tracing analysis revealed that DiI-positive motoneurons in the brainstem facial nucleus at 13 weeks after transplantation in all rats (Fig. 6), indicating that the regenerated nerves were connected to the facial nucleus by axonal flow.
Fig. 6.
Low magnified microphotographs of the rat brainstem coronal sections stained with fluorescence retrograde neuronal tracers DiI. Photograph A and B show DiI-labeled facial motoneurons in the facial nuclei of the control and dedifferentiated fat (DFAT) cell groups, respectively. Insets show high-power field view of DiI-labeled facial motor neurons. The bars in macrophotographs and the insets indicate 1 mm and 100 μm respectively.
3.3. Morphology and histology of regenerated facial nerves at 13 weeks after transplantation
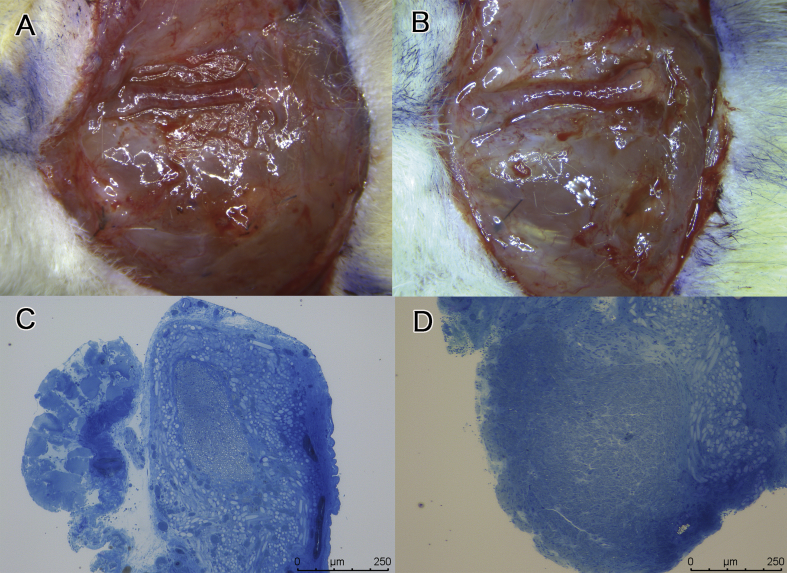
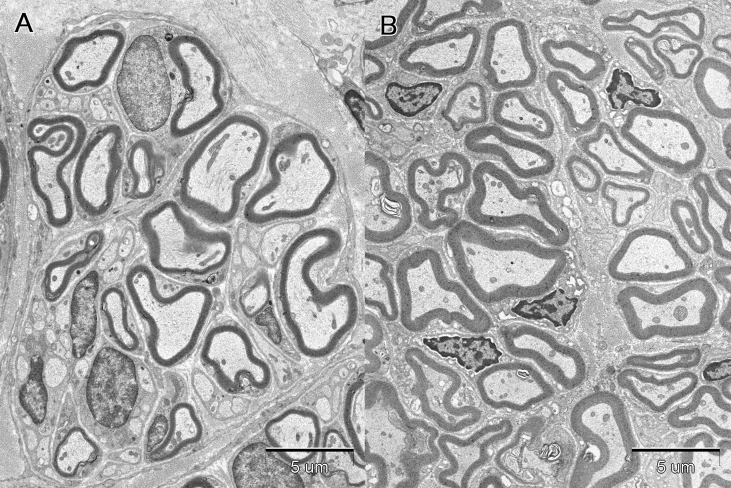
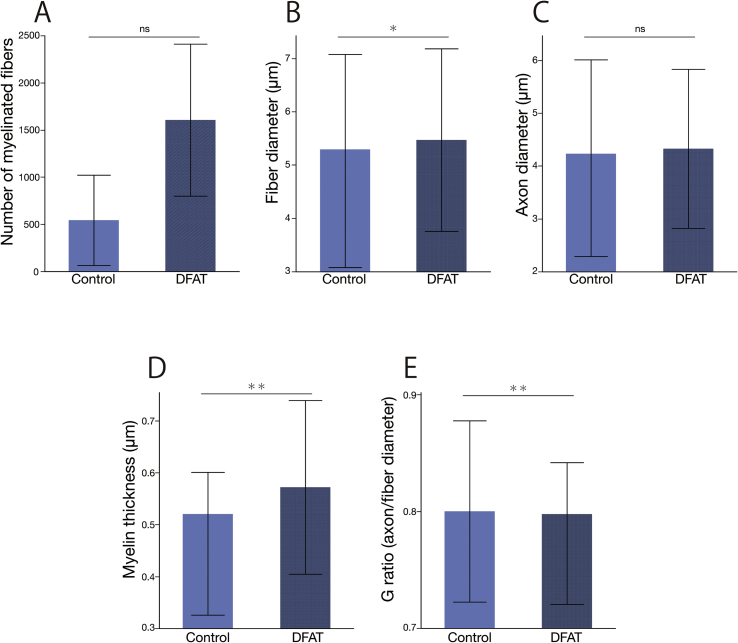
All PGA conduits were excised at 13 weeks after transplantation and showed no signs of infection. Microscopic examination showed that regenerated nerves were covered with granulation tissue in both groups (Fig. 7A and B). The central parts in the cross-sections of the regenerated nerves were observed in toluidine blue and uranyl acetate-lead citrate stained specimens with optical and electron microscopies, respectively. Toluidine blue stained specimens showed that regenerated nerve fibers were thicker in DFAT group (Fig. 7D) than in the control group (Fig. 7C). Fig. 8 shows the transmission electron microphotographs of the central parts of cross-sections of regenerated nerves at 13 weeks after transplantation. The control group (Fig. 8A) had only small thin myelin sheaths. The regenerated nerve in the dedifferentiated fat group (Fig. 8B) had very large regenerating axons and beautifully laminated myelin sheaths. From the electron microphotographs, the statistic data of the regenerated nerves were obtained (Fig. 9). The number of myelinated fibers was higher in DFAT group than in the control group (1606 ± 806 vs. 543 ± 478) (Fig. 9A). The fiber diameter was larger in DFAT group than in the control group (5.47 ± 1.71 μm vs. 5.08 ± 2.00 μm) (Fig. 9B). There were no significant differences in axon diameter between the control and DFAT groups (4.15 ± 1.86 μm vs. 4.33 ± 1.51 μm) (Fig. 9C). Myelin thickness was larger in DFAT group than in the control group (0.57 ± 0.16 μm vs. 0.46 ± 0.14 μm)
Fig. 7.
Microscopic images of the autograft and regenerated nerves in the control groups and dedifferentiated fat (DFAT) cells. Regenerated nerves were much thicker and stronger in DFAT group (B) than in the control group (A). The bars in photograph A and B indicate 5 mm. Photograph C and D shows the cross-sections of the central part of toluidine blue stained regenerated nerves. (C) In the control group, the regenerated nerve was composed of a single fascicular nerve with a few thin blood vessels coursing along the regenerated nerves with axonal regeneration appearing in a smaller area than in DFAT group. The bars indicate 250 μm. (D) In DFAT group, the regenerated nerve was composed of a single fascicular nerve with dense axonal regeneration in the regenerated nerve and thick axial vessels coursing along the epineurium.
Fig. 8.
Transmission electron microphotographs of the cross-sections of the central parts of the regenerated nerves at 13 weeks after transplantation. Microphotograph A and B show the cross-section of the nerve in the control and DFAT groups, respectively. The bar indicate 5 μm at a magnification of 3550.
Fig. 9.
Statistics of the regenerated nerves in the control and dedifferentiated fat (DFAT) cells groups. (A) The number of myelinated fibers in DFAT group was higher than the control group (1606 ± 806 vs. 543 ± 478). (B) Fiber diameter was larger in DFAT group than in the control group (5.47 ± 1.71 μm vs. 5.08 ± 2.00 μm). (C) There were no significant differences in axon diameter between the control and DFAT groups (4.15 ± 1.86 μm vs. 4.33 ± 1.51 μm). (D) Myelin thickness was greater in DFAT group than in the control group (0.57 ± 0.16 μm vs. 0.46 ± 0.14 μm). (E) G ratio, which was calculated by dividing axon diameter by fiber diameter, was lower in DFAT group than in the control group (0.78 ± 0.06 μm vs. 0.80 ± 0.08 μm). The bars above and under the columns show the standard deviations (SDs). One (*) and two asterisks (**) show probabilities of less than 0.05 (p < 0.05) and less than 0.01 (p < 0.01), respectively. The abbreviation “n.s.” indicates non-significance.
(Fig. 9D). G-ratio, which was calculated by dividing axon diameter by fiber diameter, was lower in DFAT group than in the control group (0.78 ± 0.06 μm vs. 0.80 ± 0.08 μm) (Fig. 9E).
4. Discussion
Various stem-cell transplantation techniques that can be used as a replacement for autologous nerve transplantation are studied for treating facial nerve damage caused by trauma or malignant tumor resection, and the use of DFAT cells is one of these treatment techniques. As the advantages of DFAT cells, (1) DFAT cells are generated from mature adipocytes more readily obtained without complicated cell-selection procedures than ADSC or SVF [16], (2) collection of only one gram or less of mature adipocytes is required for preparing the cells, resulting in minimizing damage to the adipocyte collection site, and (3) the large amount of the cells can be prepared in a short period of time by a conventional method. The authors previously reported that DFAT cell-filled silicone tubes promote facial nerve regeneration [17]. For aiming the clinical application of DFAT cells, this study histologically confirmed the effectiveness of DFAT cells in biodegradable artificial nerve conduits on facial nerve regeneration. At 13 weeks after transplantation, a marked promotive effect on nerve regeneration was found in DFAT group. Although physiological investigations by CMAP measurement revealed no significant difference between the control and DFAT groups, the whisker-motion experiment showed a significantly larger movement angle in DFAT group, suggesting the physiological effect of DFAT cells on promoting nerve regeneration.
The favorable promotion of nerve regeneration found in this study using conduits filled with DFAT-cells could be caused by (1) the release of growth factors from DFAT cells and (2) the pluripotency of DFAT cells. As the first reason, vascular endothelial growth factor (VEGF) is known to be released from DFAT cells in excessive quantities by protein array analysis [13]. Axonal outgrowth is stimulated by the neurotrophic activity of VEGF in cervical ganglia or dorsal ganglia [25]. Hobson et al. apply silicone tubes filled with VEGF to a 1-cm nerve defect created in a rat sciatic nerve and find that (1) Schwann cells proliferate more significantly and (2) the mean number of myelinated fibers increased by 78% in VEGF-filled conduits [26]. The effect of VEGF was therefore likely to also appear in the facial nerve in this study. As the second reason, the pluripotency of DFAT cells could contribute to nerve regeneration. Previously, double immunostaining experiment shows that green fluorescent protein-positive DFAT cells differentiate into Schwann cells in the regenerated nerves in artificial nerves conduits [17]. Schwann cells are peripheral glial cells that contribute to the maintenance of axon structure by forming myelin. Schwann cells essentially play a central role in nerve regeneration by releasing growth factors necessary for neuronal maintenance [27]. Immunostaining studies show that neural stem cell markers such as nestin and SOX2 are found in DFAT cells [28], [29], suggesting that DFAT cells differentiate into peripheral nerve cells. Several studies are known to investigate the enhancement of nerve regeneration by ADSC. Kono et al. show that DFAT and ADSC display similar immunophenotypes, but DFAT has a greater homogeneity than ADSC, because DFAT is produced by ceiling culture. In the future, the authors would like to compare the nerve regeneration ability of ADSC to that of DFAT and investigate how DFAT's homogeneity affects nerve regeneration [29].
5. Conclusions
In a rat facial nerve defect model with a 7-mm defect, a PGA conduit filled with DFAT cells significantly promoted axonal outgrowth in regenerating nerve and enhanced the maturation and physiological function, indicating that the further improvement of the functionality of PGA conduits.
Declaration of interest
None.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science KAKENHI [Grant numbers 16K11382 and 19K10016], the Hiroto Yoshioka Memorial Foundation for Medical Research, Komei Nakayama Research Scholarships, the Japan-Bangladesh Medical Association Foundation, the Tokyo Women’s Clinic Research Foundation, and the Terumo Foundation for Life Sciences and Arts.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Harii K., Asato H., Yoshimura K., Sugawara Y., Nakatsuka T., Ueda K. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Alluin O., Wittmann C., Marqueste T., Chabas J.F., Garcia S., Lavaut M.N. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials. 2009;30:363–373. doi: 10.1016/j.biomaterials.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Matsumine H., Sasaki R., Yamato M., Okano T., Sakurai H. A polylactic acid non-woven nerve conduit for facial nerve regeneration in rats. J Tissue Eng Regenerat Med. 2014;8:454–462. doi: 10.1002/term.1540. [DOI] [PubMed] [Google Scholar]
- 4.Welin D., Novikova L.N., Wiberg M., Kellerth J.O., Novikov L.N. Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res. 2008;186:315–323. doi: 10.1007/s00221-007-1232-5. [DOI] [PubMed] [Google Scholar]
- 5.Mackinnon S.E., Dellon A.L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85:419–424. doi: 10.1097/00006534-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Mligiliche N.L., Tabata Y., Kitada M., Endoh K., Okamato K., Fujimoto E. Poly lactic acid--caprolactone copolymer tube with a denatured skeletal muscle segment inside as a guide for peripheral nerve regeneration: a morphological and electrophysiological evaluation of the regenerated nerves. Anat Sci Int. 2003;78:156–161. doi: 10.1046/j.0022-7722.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Itoh S., Matsuda A., Aizawa T., Demura M., Ichinose S. Enhanced nerve regeneration through a bilayered chitosan tube: the effect of introduction of glycine spacer into the CYIGSR sequence. J Biomed Mater Res A. 2008;85:919–928. doi: 10.1002/jbm.a.31522. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu M., Matsumine H., Osaki H., Ueta Y., Tsunoda S., Kamei W. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018;26:446–455. doi: 10.1111/wrr.12665. [DOI] [PubMed] [Google Scholar]
- 9.Yagi K., Kondo D., Okazaki Y., Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–974. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Jumabay M., Matsumoto T., Yokoyama S., Kano K., Kusumi Y., Masuko T. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–575. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Ohta Y., Takenaga M., Tokura Y., Hamaguchi A., Matsumoto T., Kano K. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–886. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 12.Nur R., Fukuda N., Matsumoto T., Medet J., Kano K., Yamamoto C. Implantation of dedifferentiated fat cells ameliorates habu snake venom-induced chronic renal dysfunction in tenascin-C-deficient mice. Nephron Exp Nephrol. 2008;110:e91–e98. doi: 10.1159/000166995. [DOI] [PubMed] [Google Scholar]
- 13.Obinata D., Matsumoto T., Ikado Y., Sakuma T., Kano K., Fukuda N. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 2011;18:827–834. doi: 10.1111/j.1442-2042.2011.02865.x. [DOI] [PubMed] [Google Scholar]
- 14.Kikuta S., Tanaka N., Kazama T., Kazama M., Kano K., Ryu J. Osteogenic effects of dedifferentiated fat cell transplantation in rabbit models of bone defect and ovariectomy-induced osteoporosis. Tissue Eng A. 2013;19:1792–1802. doi: 10.1089/ten.tea.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soejima K., Kashimura T., Asami T., Kazama T., Matsumoto T., Nakazawa H. Effects of mature adipocyte-derived dedifferentiated fat (DFAT) cells on generation and vascularisation of dermis-like tissue after artificial dermis grafting. J Plast Surg Hand Surg. 2015;49:25–31. doi: 10.3109/2000656X.2014.920712. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T., Kano K., Kondo D., Fukuda N., Iribe Y., Tanaka N. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 17.Matsumine H., Takeuchi Y., Sasaki R., Kazama T., Kano K., Matsumoto T. Adipocyte-derived and dedifferentiated fat cells promoting facial nerve regeneration in a rat model. Plast Reconstr Surg. 2014;134:686–697. doi: 10.1097/PRS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 18.Oatari M., Uehara M., Shimizu F. Evaluation of the effects of a polyglycolic acid-collagen tube in the regeneration of facial nerve defects in rats. Int J Artif Organs. 2018;41:664–669. doi: 10.1177/0391398818783860. [DOI] [PubMed] [Google Scholar]
- 19.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki R., Matsumine H., Watanabe Y., Yamato M., Ando T. Anesthesia for research on reconstructive facial surgery in rats. J Reconstr Microsurg. 2013;29:209–210. doi: 10.1055/s-0032-1331147. [DOI] [PubMed] [Google Scholar]
- 21.Matsumine H., Numakura K., Tsunoda S., Wang H., Matsumine R., Climov M. Adipose-derived aldehyde dehydrogenase-expressing cells promote dermal regenerative potential with collagen-glycosaminoglycan scaffold. Wound Repair Regen. 2017;25:109–119. doi: 10.1111/wrr.12494. [DOI] [PubMed] [Google Scholar]
- 22.Matsumine H., Sasaki R., Takeuchi M., Yamato M., Sakurai H. Surgical procedure for transplanting artificial nerve conduits for peripheral nerve regeneration. Plast Reconstr Surg. 2011;128:95e–97e. doi: 10.1097/PRS.0b013e31821ef380. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi R., Sanaei N., Ahsan S., Masoumi-Verki M., Khadir F., Mokarizadeh A. Stromal vascular fraction combined with silicone rubber chamber improves sciatic nerve regeneration in diabetes. Chin J Traumatol. 2015;18:212–218. doi: 10.1016/j.cjtee.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K., Ohnishi K., Kiyotani T., Sekine T., Ueda H., Nakamura T. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]
- 25.Sondell M., Lundborg G., Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobson M.I., Green C.J., Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197 Pt 4:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall S. Nerve repair: a neurobiologist's view. J Hand Surg Br. 2001;26:129–136. doi: 10.1054/jhsb.2000.0497. [DOI] [PubMed] [Google Scholar]
- 28.Kakudo T., Kishimoto N., Matsuyama T., Momota Y. Functional recovery by application of human dedifferentiated fat cells on cerebral infarction mice model. Cytotechnology. 2018;70:949–959. doi: 10.1007/s10616-018-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kono S., Kazama T., Kano K., Harada K., Uechi M., Matsumoto T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet J. 2014;199:88–96. doi: 10.1016/j.tvjl.2013.10.033. [DOI] [PubMed] [Google Scholar]