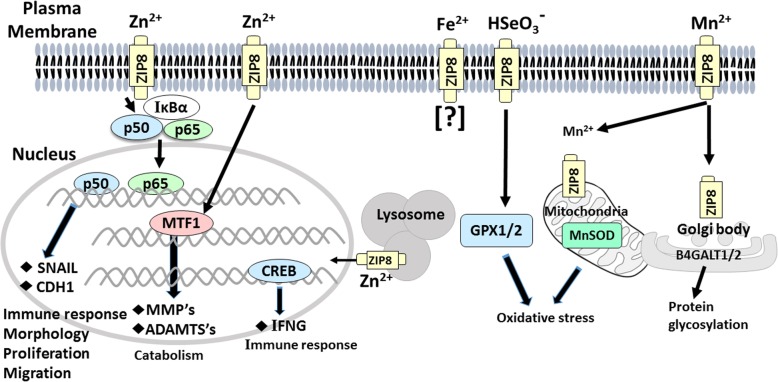
Fig. 2.
Molecular mechanisms of ZIP8 transport function and related downstream pathways in various cell organelles. At far left, ZIP8 imports Zn2+; a cofactor, in the NFκB subunit P65, then inactivates NFκB. Downstream targets SNAIL and CDH1 participate in the immune response, cell morphology, proliferation, and migration. Increased levels of intracellular Zn2+ can also activate MTF1 which, in cartilage, enhances catabolic processes—including matrix metallopeptidases (MMP’s) and ADAM metallopeptidases with thrombospondin types (ADAMTS’s) that hydrolyze proteins. At left center, Zn2+ influx by ZIP8 in the lysosomal membrane elevates cAMP-responsive element-binding protein (CREB), which regulates interferon-γ (INFG) expression involved in the immune response. At center, any role for ZIP8-mediated Fe2+ uptake has not been studied to date (denoted by the “?”). At right center, ZIP8-mediated selenite [(HSeO3)−] influx likely affects activities of selenoproteins such as glutathione peroxidases-1, -2 (GPX1/2). At far right, ZIP8-mediated Mn2+ uptake is critical for Mn-dependent enzymes such as mitochondrial manganese-superoxide dismutase (MnSOD) in mitochondria; decreases in both GPX1/2 and MnSOD result in increased oxidative stress. Deficiencies in Mn-dependent enzymes—including β-1,4-galactosyltransferases-1, -2 (B4GALT1/2) in the Golgi body—results in defects in posttranslational glycosylation and almost half of all proteins synthesized in the cell. See text and references cited therein for details