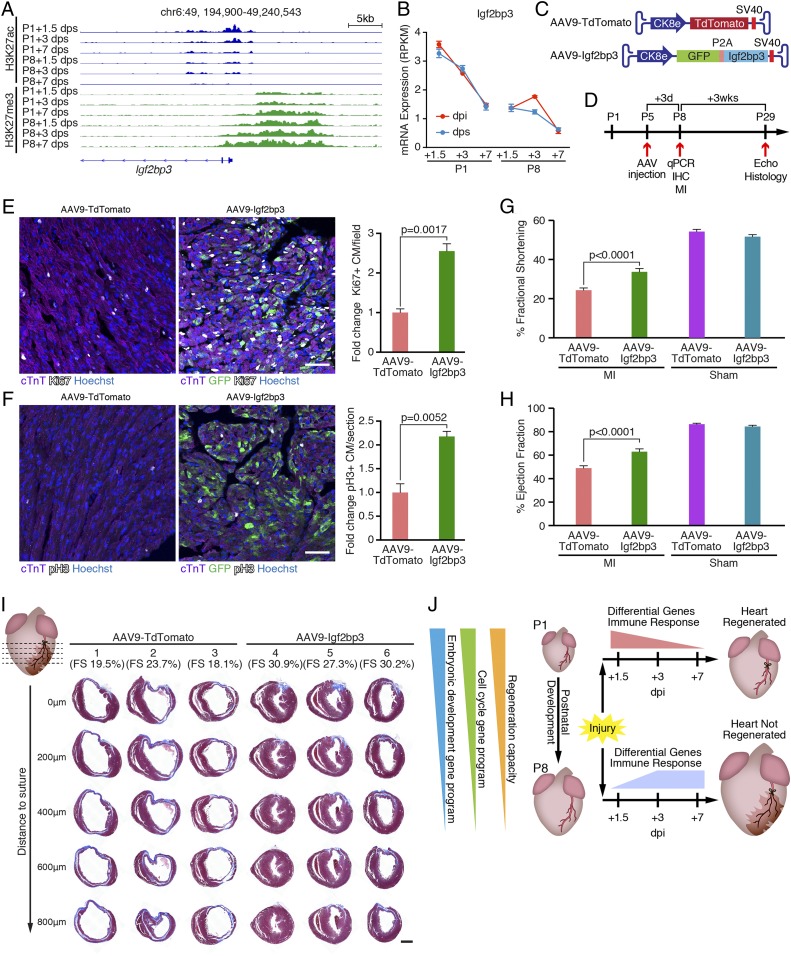
Fig. 5.
Igf2bp3 is highly expressed in regenerative hearts and promotes CM proliferation. (A) Decreasing H3K27ac and increasing H3K27me3 signals at the Igf2bp3 genomic locus in sham control hearts during postnatal development. chr, chromosome. (B) Igf2bp3 mRNA expression in hearts decreases from P1 to P8, and is not induced by MI (n = 3 for each condition). RPKM, reads per kilobase of transcript per million mapped reads. (C) Illustration of AAV9 vectors for overexpressing TdTomato (AAV9-TdTomato, control) and Igf2bp3 (AAV9-Igf2bp3). Ck8e, muscle creatine kinase promoter; P2A, porcine teschovirus-1 2A self-cleaving peptide coding sequence; SV40, simian virus 40 poly-A signal. (D) Strategy of AAV9 injection experiment. C57BL6 male mice were injected with AAV9-TdTomato or AAV9-Igf2bp3 at P5. At 3 d postinjection (P8), some mice (randomly selected) were euthanized for immunohistochemistry (IHC) analysis of proliferating CMs or qPCR analysis to examine Igf2bp3 overexpression. The remaining mice were subjected to MI surgery at P8, their cardiac function was examined by echocardiography (Echo) 3 wk post-MI (P29), and they were euthanized for histological examination. (E, Left) Immunostaining of cTnT (purple), Ki67 (white), and GFP (green) proteins on transverse sections from AAV9-TdTomato and AAV9-Igf2bp3 hearts at P8. Nuclei were counterstained with Hoechst (blue). (Scale bar, 50 μm.) (E, Right) Quantification of immunostaining images showing the fold change of Ki67+/cTnT+ double-positive CM percentage in AAV9-Igf2bp3 hearts compared with AAV9-TdTomato hearts (n = 3 for each group). (F, Left) Immunostaining of cTnT (purple), pH3 (white), and GFP (green) proteins on transverse sections from AAV9-TdTomato and AAV9-Igf2bp3 hearts at P8. Nuclei were counterstained with Hoechst (blue). (Scale bar, 50 μm.) (F, Right) Quantification of immunostaining images showing the fold change of pH3+/cTnT+ double-positive CM percentage in AAV9-Igf2bp3 hearts compared with AAV9-TdTomato hearts (n = 3 for each group). (G) Fractional shortening measurements of mice injected with AAV9-TdTomato (control) or AAV9-Igf2bp3 at P5 and analyzed at 3 wk after P8 MI, measured by echocardiography (n = 20 for AAV9-TdTomato MI group, n = 22 for AAV9-Igf2bp3 MI group, n = 2 for AAV9-TdTomato sham group, and n = 6 for AAV9-Igf2bp3 sham group). (H) Ejection fraction measurements of mice injected with AAV9-TdTomato (control) or AAV9-Igf2bp3 at P5 and analyzed at 3 wk after P8 MI, measured by echocardiography (n = 20 for AAV9-TdTomato MI group, n = 22 for AAV9-Igf2bp3 MI group, n = 2 for AAV9-TdTomato sham group, and n = 6 for AAV9-Igf2bp3 sham group). (I) Masson’s trichrome staining showing AAV9-Igf2bp3 promotes heart regeneration. P29 hearts injected with AAV9-TdTomato or AAV9-Igf2bp3 were transversely sectioned at the level of LAD ligation (0 μm), and at 200, 400, 600, and 800 μm below the suture. Three representative hearts are shown for each group, and their fractional shortening (FS) measurements are noted. (Scale bar, 500 μm.) (J) Model showing that both P1-unique injury responses (differential gene usage and distinct immune response dynamics) and the intrinsic properties of the regenerative heart (retained cell cycle activity and embryonic developmental gene program) define neonatal mouse heart regeneration.