Abstract
Purpose
To investigate the utility of mammography for breast cancer screening in a population of males at increased risk for breast cancer.
Methods
In this HIPAA-compliant institutional review board-approved single-institution study, mammography records and clinical data of 827 male patients who underwent digital mammography from September 2011–July 2018 were analyzed via the electronic medical record. 664 of these men presented with masses, pain, or nipple discharge and were excluded from this study. The remaining 163 asymptomatic men with familial and/or personal history of breast cancer, or with a known germline mutation in BRCA underwent screening mammography and were included in this analysis.
Results
163 asymptomatic men (age: mean 63 years, range 24–87 years) underwent 806 screening mammograms. 125/163 (77%) had a personal history of breast cancer and 72/163 (44%) had a family history of breast cancer. 24/163 (15%) were known mutation carriers: 4/24 (17%) BRCA1 and 20/24 (83%) BRCA2. 792/806 (98%) of the screening mammograms were negative (BI-RADS 1 or 2); 10/806 (1.2%) were classified as BI-RADS 3, all of which were eventually downgraded to BI-RADS 2 on follow-up. 4/806 (0.4%) mammograms were abnormal (BI-RADS 4/5): all were malignant. The cancer detection rate in this cohort was 4.9 cancers/1,000 examinations.
Conclusions
In our cohort, screening mammography yielded a cancer detection rate of 4.9 cancers/1000 examinations which is like the detection rate of screening mammography in a population of women at average risk, indicating that screening mammography is of value in male patients at high risk for breast cancer.
Keywords: digital mammography, breast cancer, male, screening, diagnosis
INTRODUCTION
Male breast cancer (BC) represents < 0.5% of cancer diagnoses in men and 1% of all breast cancers in the United States [1]. The current understanding of male BC is limited to the findings from small retrospective series. Men have been underrepresented in clinical trials, and the treatment recommendations for male patients have largely followed those for postmenopausal women [2]. Up to 20% of men who have been diagnosed with BC have a reported family history of breast and ovarian cancer, approximately 10% have a BRCA2 mutation, and fewer have a BRCA1 mutation [3, 4]. Several other genetic disorders, such as Klinefelter’s syndrome, can increase breast cancer risk in men by 50-fold [5]. The American Society of Clinical Oncology (ASCO) recommends that all men with BC should be offered genetic counseling and testing, regardless of family history [2].
The clinical course of male BC may be different from that in women possibly due to differences in genetic signatures and tumor biology [6–8]. While approximately 60% of female BCs are hormone receptor positive, 90% of male BCs are hormone receptor positive, most commonly estrogen (ER)-positive, progesterone (PR)-positive, and human epidermal growth factor receptor 2 (HER2)–negative. Less than 0.5% of male BCs are triple-negative BCs, compared with the 10%–15% in the female population. Similarly, male BCs are predominantly ductal in origin, and lobular cancers are rare (< 0.5%) because the male breast intrinsically lacks lobules [10]. Invasive papillary carcinoma represents a higher proportion of cancers in males than in females, accounting for approximately 2% to 4% of BCs in men compared with only 1% in women [2].
More men will die of BC (480 deaths in 2550 cases) than of testicular cancer (400 deaths in 9310 cases) in the United States in 2018, according to the estimates of the American Cancer Society [9]. In theory, as with women, earlier diagnosis of male BC should provide considerable improvement in clinical outcomes. While tamoxifen treatment has been shown to be beneficial for ER-positive BC, not all male patients with ER-positive BC receive tamoxifen treatment [10].
The current National Comprehensive Cancer Network guidelines [11] for male carriers of BRCA1 or BRCA2 mutations include (a) annual clinical breast examination and self-examination to begin at 35 years old and (b) prostate cancer screening to begin at 45 years old. Mammographic screening is not recommended presumably owing to the limited data supporting imaging in men. There are also no screening recommendations for men with a personal history of breast cancer or those with strong family histories. Therefore, the aim of our study was to investigate the utility of mammography for breast cancer screening in a population of men at increased risk for breast cancer.
MATERIAL AND METHODS
Our institutional review board approved this single-institution retrospective study, which was HIPAA compliant. The need for informed consent was waived.
Patients
A search of a prospectively populated database from September 2011 to July 2018 yielded 827 male patients who underwent digital mammography at our tertiary cancer center. 664/827 patients (80%) undergoing mammography were excluded because they had undergone diagnostic mammography due to symptoms or palpable abnormalities on physical exam.
The remaining 163 patients were the subjects of this study.
Patient and Tumor Characteristics
The following patient and tumor characteristics were recorded: risk factors for developing cancer, including personal history, family history, any documented genetic mutation, age at first screening, age at diagnosis, tumor histology, nuclear grade, receptor status, tumor size, history of gynecomastia, personal history of other cancer, stage of cancer, race/ethnicity, and prior radiation treatment.
Imaging
Standard mammography included two views per breast with additional views performed at the discretion of the interpreting radiologist. If necessary, patients also underwent targeted breast ultrasound. Mammographic interpretation was reported using the American College of Radiology BI-RADS mammography lexicon [12].
Statistical Analysis
Descriptive statistics were calculated for patients’ demographic and clinical characteristics. Frequencies and percentages were used to summarize the distribution of histology, tumor receptor status, nuclear grade, and histological grade. Imaging scores of Breast Imaging Reporting and Data System (BI-RADS) 3, 4, or 5 were considered to be a positive screening result. We calculated the detection rates of screen-detected cancers, interval-detected cancers, and their sum (that is, the 2-year breast cancer incidence rates) per 1,000 men screened
RESULTS
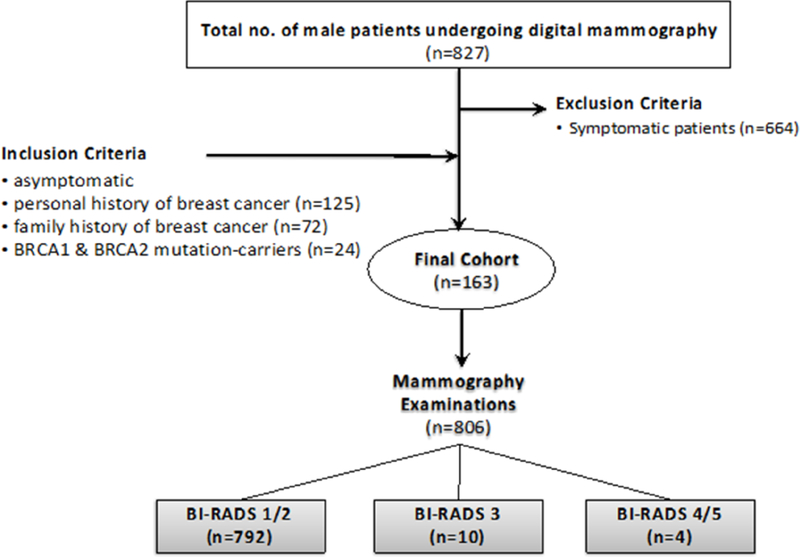
The study population comprised 163 asymptomatic men (mean age 63 years; range 24–87 years) with familial and/or personal history of breast cancer or with a known germline mutation in BRCA. Namely, 125/163 (77%) had a personal history of breast cancer and 72/163 (44%) had a family history of breast cancer. 24/163 (15%) were known mutation carriers: 4/24 (17%) BRCA1 and 20/24 (83%) BRCA2 (Figure 1).
Fig. 1.
Recruitment flowchart showing the final cohort, number of mammographic screening rounds, and BI-RADS assessment categories over the screening time period.
Table 1 demonstrates patient characteristics stratified by risk factors.
Table 1:
Characteristics of participant men
| n | % | |
|---|---|---|
| RACE/ETHNICITY | 163 | 100 |
| White not Hispanic | 132 | 81.0 |
| Black or African American | 10 | 6.1 |
| Other | 7 | 4.3 |
| Ashkenazi Jewish | 6 | 3.7 |
| Asian-Far East/Indian Subcontinent | 5 | 3.1 |
| Hispanic or Latino | 3 | 1.8 |
| FAMILIAL HISTORY OF BREAST CANCER | 72 | 44.2 |
| GENETIC MUTATION | 24 | 14.7 |
| BRCA1 mutation carrier | 4 | 16.7 |
| BRCA2 mutation carrier | 20 | 83.3 |
| PERSONAL HISTORY OF BREAST CANCER | 125 | 76.7 |
| PRIOR RADIATION TREATMENT | 12 | 7.4 |
| HISTORY OF OTHER CANCER | 42 | 25.7 |
| Prostate | 24 | 57.1 |
| Hematologic | 5 | 12 |
| Gastrointestinal | 4 | 9.5 |
| Bladder | 2 | 4.7 |
| Sarcoma | 2 | 4.7 |
| Skin | 2 | 4.7 |
| Other | 3 | 7.1 |
| GYNECOMASTIA | 70 | 43.0 |
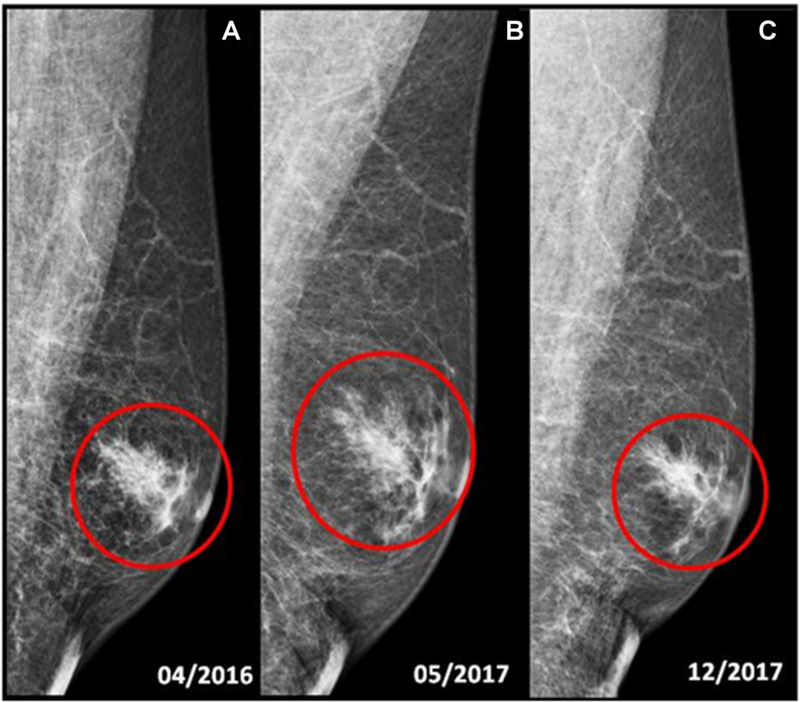
The 163 men underwent 806 screening mammography examinations. There were 792/806 (98%) negative mammograms (BI-RADS 1 or 2). 10/806 (1.2%) of the mammograms were classified as BI-RADS 3. Three mammographic examinations (3/10, 30%) were assessed as BI-RADS 3 (probably benign) for focal asymmetry. Two men (2/3, 67%) underwent a twelve-month follow-up and one a six-month follow-up. All abnormalities were determined to be gynecomastia (Figure 2). Three mammograms were considered BI-RADS 3 for post-surgical architectural distortion. None of those men underwent biopsy and all were downgraded to BI-RADS 2. Finally, two cases of BI-RADS 3 were due to lymph-nodes: in one man with previous history of breast cancer, an enlarged axillary lymph-node was found and at the six-month follow-up, this was considered benign (BI-RADS 2). In another, a probable intramammary lymph node was detected, which was stable and considered benign (BI-RADS 2) at 6-month follow-up. Details of all patients with a positive screening mammogram are shown in Table 2.
Fig. 2.
69-year-old male with family history of breast cancer, tested positive for BRCA2 gene mutation. (a, b, c) Screening mammography of the left breast, medio-lateral oblique (MLO) view, shows predominantly fatty breasts. (a) No suspicious mass or tumor calcifications are seen. Mild asymmetric gynecomastia is present (red circle). BI-RADS 2: benign. Routine annual screening mammography is recommended. (b) There is increasing gynecomastia (red circle), not palpable, for which six month follow-up is recommended. BI-RADS 3: Probably benign. 6 months left breast follow-up is recommended. (c) Left mammogram follow-up. No suspicious mass or tumor calcifications are seen. Left gynecomastia is unchanged (red circle). BI-RADS 2: BENIGN. Routine annual screening mammography is recommended.
Table 2:
Details of patients with a positive screening mammogram
| CASE NUMBER | BI-RADS | REPORTED ABNORMALITY | MANAGEMENT | RESULTS |
|---|---|---|---|---|
| 7 | 3 | Increased left gynecomastia | 6 MO FU | BIRADS 2 |
| 47 | 4/5 | New mass | BX | IDC |
| 55 | 4/5 | New mass | BX | IDC |
| 60 | 4/5 | New mass | BX | IDC |
| 62 | 3 | Post-surgical architectural distortion | 6 MO FU | BIRADS 2 |
| 63 | 4/5 | New mass | BX | IDC |
| 76 | 3 | enlarged lymph node in the axilla | 6 MO FU | BIRADS 2 |
| 84 | 3 | Architectural Distortion | 12 MO FU | BI-RADS 2 |
| 112 | 3 | Focal asymmetry | 12 MO FU | BI-RADS 2 (gynecomastia) |
| 119 | 3 | Post-surgical Architectural distortion | 6 MO FU | BI-RADS 2 |
| 131 | 3 | Intra-mammary lymph node | 6 MO FU | BI-RADS 2 |
| 133 | 3 | Focal asymmetry | 12 MO FU | BI-RADS 2 (gynecomastia) |
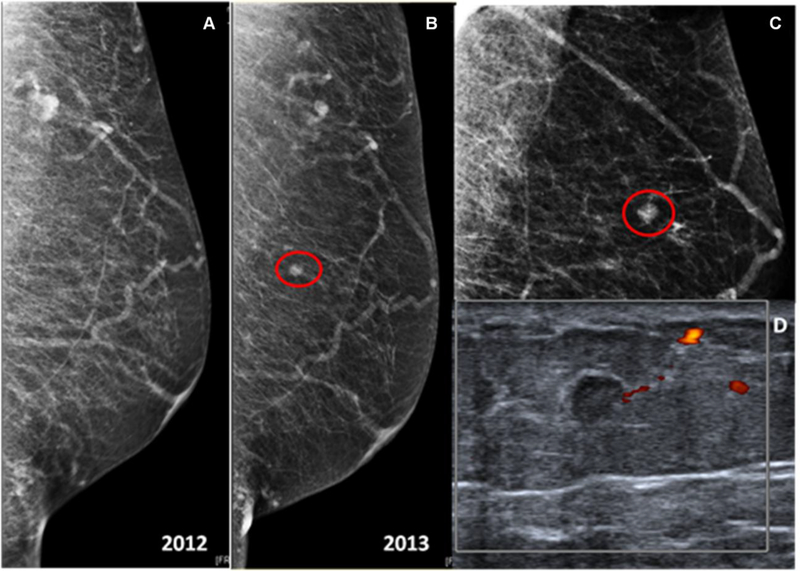
4/806 (0.4%) mammograms in these asymptomatic men were suspicious (BI-RADS 4/5) and underwent biopsy yielding invasive ductal carcinoma (mean size 6.25 mm, range 4–7mm) (PPV3 100%) (Figure 3). They were all node negative patients.
Fig. 3.
66-year-old male with prior post-right mastectomy for breast cancer. (a) Routine annual screening of the left breast, mediolateral-oblique (MLO) view. The breast is predominantly fatty without any suspicious mass or tumor calcification (BI-RADS 1: Negative). (b, c) The following year, the left mammogram, mediolateral-oblique and spot projections, demonstrates in the upper-inner quadrant, middle third depth, a new 0.7 cm round mass. (d) The targeted left breast ultrasound demonstrates in the 11:00 axis, 8 cm from the nipple, a 0.5 cm irregular, anti-parallel, hypoechoic mass with no significant vascularization at color doppler ultrasound. This appears to represent a mammographic correlate. Final BI-RADS score 4C: High suspicion for malignancy. Ultrasound-guided biopsy is recommended. Pathology yielded invasive ductal carcinoma, moderately differentiated with focal necrosis, solid growth pattern, and papillary features (Estrogen Receptor 95%, Progesterone Receptor 70%, Her-2/neu negative).
The cancer detection rate was 4.9 per 1,000 mammograms.
None of the screening participants developed an interval cancer.
DISCUSSION
BC continues to be a leading cause of cancer death in women. While mammographic screening has been shown to be useful in the reduction of BC mortality in women, this has not yet been demonstrated in men. Therefore, although there are screening guidelines proposed for women [13–23], there are currently no screening guidelines for men even among men with known risk factors for developing breast cancer. The paucity of literature concerning breast cancer in men, which predominantly consists of small retrospective studies, have led to a limited understanding of possible screening strategies. To our knowledge, there are no large studies evaluating the use of screening mammography in high risk men: Brenner et al. [24] reported a case of a carcinoma detected as a nodule on a screening mammogram of a 65-year-old man, with a history of breast cancer in the contralateral breast, and a diagnosis of BRCA2 mutation. Dershaw et al. [25, 26] reported breast carcinoma in the contralateral breast of two men who were undergoing annual screening mammography after a mastectomy for BC. No information of the BRCA mutation status was reported. Freedman et al. [27] reported a case of a 57-year-old BRCA2 mutation carrier man with history of right breast cancer who at the second annual screening mammogram presented with fine pleomorphic microcalcifications in the retro-areolar region of the contralateral breast. Histologic evaluation identified a 9 mm multifocal ductal carcinoma in situ, solid and cribriform, and high nuclear grade without invasive component. Like our cohort, the patients described in the above reports were asymptomatic and the breast cancers were detected by screening mammogram. Our study showed that screening mammography in men at increased risk for breast cancer yielded a cancer detection rate of 4.9/1000 which is similar to that of screening mammography in women (5.4/1000) in population based screening [23]. This finding suggests that screening mammography has potential utility in cancer detection in men at higher risk for developing BC.
Breast cancers in men often present at a more advanced stage than breast cancers in women, with up to 47% of men having axillary nodal involvement at the time of diagnosis [28]. Anderson et al. in a population-based comparison of 5494 male BC and female BC [29] demonstrated that the biology of male BC resembled that of late-onset female BC; that there are similar BC risk factors, especially for ER–positive BC; and that BC mortality and survival rates have improved significantly over time for both men and women, but progress for men has lagged behind that for women. The ability of a BC to metastasize to distant organs is related to its size, as well as other factors such as its intrinsic biology [30–35]. In our cohort of screen detected male BC, we found only sub-centimeter cancer without nodal involvement (stage I) suggesting that just as in women, tumor stage can be downgraded by screening with potential for improvement in overall outcomes. [36].
The American College of Radiology Appropriateness Criteria Committee recently recommended criteria for imaging the breasts in symptomatic men. [37]. The panel recommends mammography or digital breast tomosynthesis in men age 25 and older if there are symptoms or if physical examination is suspicious for BC. In this study we showed that in asymptomatic men at high risk for developing BC, mammography can be useful as well. There were only 10/806 (1.2%) BI-RADS 3 cases well within the guidelines for women and no false positive biopsy recommendations.
We acknowledge the limitations of our study. The biggest limitation is that it is a retrospective study and therefore could suffer from selection bias. Furthermore, this is a single-center study in a tertiary referral center with a large, high-risk screening program. Furthermore, we included patients at high risk for BC without stratifying for risk factors such as germline mutation status or prior history of breast cancer due to the relatively small number in some subgroups. The numbers of participants and cancers detected were still small, leading to potential issues with statistical power, precision, and validity. Additional studies with larger cohorts are required to confirm our findings.
In conclusion, our study suggests that screening mammography should be performed in men at increased risk for BC. Larger populations of high-risk men should be studied to validate these results.
Acknowledgments
FUNDING INFORMATION:
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 and a grant from the Breast Cancer Research Foundation. The sponsors were not involved in the study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the article for publication.
CONFLICT OF INTEREST:
KP received payment for activities not related to the present article including lectures including service on speakers bureaus and for travel/ accommodations/meeting expenses unrelated to activities listed from the European Society of Breast Imaging (MRI educational course, annual scientific meeting). MSJ has received an honorarium from GE for speaking, and an honorarium for speaking at the Lynn Sage Breast Cancer Symposium and at MD Anderson. EAM has received a grant from GRAIL. The rest of the authors declare that they have no conflict of interest.
Footnotes
ETHICAL APPROVAL:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
INFORMED CONSENT:
As this was a retrospective study, the need for informed consent was waived.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Liu N, Johnson KJ, Ma CX (2018) Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin Breast Cancer 18(5):e997–e1002. 10.1016/j.clbc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Korde LA, Zujewski JA, Kamin L, et al. (2010) Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 28:2114–2122. 10.1200/JCO.2009.25.5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liede A, Karlan BY, Narod SA (2004) Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 22:735–742. 10.1200/JCO.2004.05.055 [DOI] [PubMed] [Google Scholar]
- 4.Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31:55–59. 10.1038/ng879 [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Crichlow RW (1987) Carcinoma of the male breast and Klinefelter’s syndrome: is there an association? CA Cancer J Clin 37:246–251 [DOI] [PubMed] [Google Scholar]
- 6.Yu X-F, Feng W-L, Miao L-L, et al. (2013) The prognostic significance of molecular subtype for male breast cancer: a 10-year retrospective study. Breast 22:824–827. 10.1016/j.breast.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Piscuoglio S, Ng CKY, Murray MP, et al. (2016) The Genomic Landscape of Male Breast Cancers. Clin Cancer Res 22:4045–4056. 10.1158/1078-0432.CCR-15-2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen MA, Slaets L, Cardoso F, et al. (2017) Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer 82:219–227. 10.1016/j.ejca.2017.01.034 [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, Bartlett JMS, Slaets L, et al. (2018) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 29:405–417. 10.1093/annonc/mdx651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN clinical practice guidelines in oncology (NCCN guidelines): Genetic/familial high-risk assessment—breast and ovarian, version 1.2018https://www.trikobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. (n.d.).
- 12.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/BIRADS/BIRADS%20V5%20Changes.pdf. Accessed 20 Sep 2015 [Google Scholar]
- 13.Eccles DM, Evans DG, Mackay J (2000) Guidelines for a genetic risk based approach to advising women with a family history of breast cancer. UK Cancer Family Study Group (UKCFSG). J Med Genet 37:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardanelli F, Boetes C, Borisch B, et al. (2010) Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316. 10.1016/j.ejca.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 15.Vasen HF, Haites NE, Evans DG, et al. (1998) Current policies for surveillance and management in women at risk of breast and ovarian cancer: a survey among 16 European family cancer clinics. European Familial Breast Cancer Collaborative Group. Eur J Cancer 34:1922–1926 [DOI] [PubMed] [Google Scholar]
- 16.Evans DGR, Lalloo F (2002) Risk assessment and management of high risk familial breast cancer. J Med Genet 39:865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisinger F, Alby N, Bremond A, et al. (1998) Recommendations for medical management of hereditary breast and ovarian cancer: the French National Ad Hoc Committee. Ann Oncol 9:939–950 [DOI] [PubMed] [Google Scholar]
- 18.Warner E, Heisey RE, Goel V, et al. (1999) Hereditary breast cancer. Risk assessment of patients with a family history of breast cancer. Can Fam Physician 45:104–112 [PMC free article] [PubMed] [Google Scholar]
- 19.Singer CF, Tea M-K, Pristauz G, et al. (2012) [Guideline for the prevention and early detection of breast and ovarian cancer in high risk patients, particularly in women from HBOC (hereditary breast and ovarian cancer) families]. Wien Klin Wochenschr 124:334–339. 10.1007/s00508-012-0173-6 [DOI] [PubMed] [Google Scholar]
- 20.Møller P, Evans G, Haites N, et al. (1999) Guidelines for follow-up of women at high risk for inherited breast cancer: consensus statement from the Biomed 2 Demonstration Programme on Inherited Breast Cancer. Dis Markers 15:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhl CK, Schrading S, Leutner CC, et al. (2005) Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 23:8469–8476. 10.1200/JCO.2004.00.4960 [DOI] [PubMed] [Google Scholar]
- 22.Leach MO, Boggis CRM, Dixon AK, et al. (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365:1769–1778. 10.1016/S0140-6736(05)66481-1 [DOI] [PubMed] [Google Scholar]
- 23.Kuhl C, Weigel S, Schrading S, et al. (2010) Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 28:1450–1457. 10.1200/JCO.2009.23.0839 [DOI] [PubMed] [Google Scholar]
- 24.Brenner RJ, Weitzel JN, Hansen N, Boasberg P (2004) Screening-detected breast cancer in a man with BRCA2 mutation: case report. Radiology 230:553–555. 10.1148/radiol.2302030360 [DOI] [PubMed] [Google Scholar]
- 25.Dershaw DD (1986) Male mammography. AJR Am J Roentgenol 146:127–131. 10.2214/ajr.146.1.127 [DOI] [PubMed] [Google Scholar]
- 26.Dershaw DD, Borgen PI, Deutch BM, Liberman L (1993) Mammographic findings in men with breast cancer. AJR Am J Roentgenol 160:267–270. 10.2214/ajr.160.2.8424331 [DOI] [PubMed] [Google Scholar]
- 27.Freedman BC, Keto J, Rosenbaum Smith SM (2012) Screening mammography in men with BRCA mutations: is there a role? Breast J 18:73–75. 10.1111/j.1524-4741.2011.01185.x [DOI] [PubMed] [Google Scholar]
- 28.Mathew J, Perkins GH, Stephens T, et al. (2008) Primary Breast Cancer in Men: Clinical, Imaging, and Pathologic Findings in 57 Patients. Am J Roentgenol 191:1631–1639. 10.2214/AJR.08.1076 [DOI] [PubMed] [Google Scholar]
- 29.Anderson WF, Jatoi I, Tse J, Rosenberg PS (2010) Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 28:232–239. 10.1200/JCO.2009.23.8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigelt B, Peterse JL, van ‘t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5:591–602. 10.1038/nrc1670 [DOI] [PubMed] [Google Scholar]
- 31.Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer 3:453–458. 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- 32.Hellman S (1994) Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol 12:2229–2234. 10.1200/JCO.1994.12.10.2229 [DOI] [PubMed] [Google Scholar]
- 33.Michaelson JS, Silverstein M, Wyatt J, et al. (2002) Predicting the survival of patients with breast carcinoma using tumor size. Cancer 95:713–723. 10.1002/cncr.10742 [DOI] [PubMed] [Google Scholar]
- 34.Gibbs P, Onishi N, Sadinski M, et al. (2019) Characterization of Sub-1 cm Breast Lesions Using Radiomics Analysis. J Magn Reson Imaging JMRI. 10.1002/jmri.26732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sopik V, Narod SA (2018) The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat 170:647–656. 10.1007/s10549-018-4796-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mango V, Bryce Y, Morris EA, et al. (2018) Commentary ACOG Practice Bulletin July 2017: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Br J Radiol 91:20170907 10.1259/bjr.20170907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Expert Panel on Breast Imaging:, Niell BL, Lourenco AP, et al. (2018) ACR Appropriateness Criteria® Evaluation of the Symptomatic Male Breast. J Am Coll Radiol JACR 15:S313–S320. 10.1016/j.jacr.2018.09.017 [DOI] [PubMed] [Google Scholar]