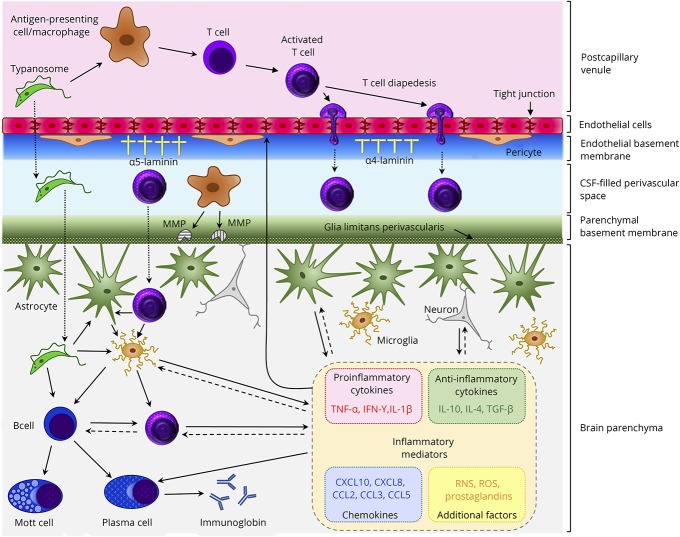
Figure. Purported mechanisms in the neuropathogenesis of HAT.
In the healthy individual, a limited number of T cells enter the brain to carry out immune surveillance. As HAT advances, lymphocytes and parasites migrate from postcapillary venules across the BBB to enter the brain parenchyma. The mechanisms facilitating this progression and those controlling the ensuing neuroinflammatory reaction are not fully understood; however, some aspects have been elucidated. The production of the cysteine protease, brucipain, by the parasites increases their ability to migrate across the BBB endothelial cell layer. After activation in the peripheral compartment, T-cell diapedesis through the endothelial cell layer of the barrier can occur using both paracellular and transcellular routes. On breaching the endothelial cell layer, both T-cells and trypanosomes must penetrate the endothelial basement membrane. This is dependent on the laminin subtypes present. Areas where α4 laminin predominates allow transmigration while α5 laminin-rich regions inhibit crossing into the perivascular space. Before reaching the brain parenchyma, T cells and parasites must traverse the parenchymal basement membrane. This step appears to be dependent on a number of factors including the presence of IFN-γ and the production of MMPs, most likely by perivascular macrophages, which disengage the astrocyte end-feet from the glial limitans allowing the cells to enter the brain. The T cells can then interact with resident microglia, astrocytes, or additional lymphocytes that can each produce a range of inflammatory mediators. The overall balance of these mediators is critical in controlling the neuroinflammatory response and BBB integrity and may prompt clinical manifestations of the disease including pyrexia, cachexia, and sleep disturbances. BBB = blood-brain barrier; HAT = human African trypanosomiasis; MMP = matrix metalloproteinases.