Abstract
Mesenchymal stromal cells (MSCs) are key elements in the bone marrow (BM) niche where they interact with hematopoietic stem progenitor cells (HSPCs) by offering physical support and secreting soluble factors, which control HSPC maintenance and fate. Although necessary for their maintenance, MSCs are a rare population in the BM, they are plastic adherent and can be ex vivo expanded to reach numbers adequate for clinical use. In light of HSPC supportive properties, MSCs have been employed in phase I/II clinical trials of hematopoietic stem cell transplantation (HSCT) to facilitate engraftment of hematopoietic stem cells (HSCs). Moreover, they have been utilized to expand ex vivo HSCs before clinical use. The available clinical evidence from these trials indicate that MSC administration is safe, as no acute and long-term adverse events have been registered in treated patients, and may be efficacious in promoting hematopoietic engraftment after HSCT. In this review, we critically discuss the role of MSCs as component of the BM niche, as recent advances in defining different mesenchymal populations in the BM have considerably increased our understanding of this complex environment. Moreover, we will revise published literature on the use of MSCs to support HSC engraftment and expansion, as well as consider potential new MSC application in the clinical context of ex vivo gene therapy with autologous HSC.
Introduction
Human bone marrow (BM) niche includes several nonhematopoietic cells, including mesenchymal stromal cells (MSCs), osteoblasts, adipocytes, and endothelial and neural cells that offer physical support to hematopoietic stem progenitor cells (HSPCs) and regulate their homeostasis. In particular, MSCs are crucial elements of the BM niche where they provide newly formed osteoblasts for bone tissue regeneration and tightly control HSPC fate by direct interaction and through the secretion of soluble factors, thus playing a key role in the development and differentiation of the hematopoietic system.1–3 MSCs were first described in 1968 by Friedenstein as a population of adherent cells present in the BM, which exhibited a fibroblast-like morphology and the ability to differentiate in vitro into mesodermal lineages.4 Due to the low frequency of mesenchymal progenitors in the human BM, where they only occur for approximately 0.001% to 0.01% of BM mononuclear cells (MNCs), ex vivo expansion of MSCs is necessary to reach sufficient numbers for clinical use.5,6 To this aim, standardized techniques are available to isolate and expand MSCs from MNCs or CD34 negative fraction of BM aspirates, including the use of culture media added with either fetal calf serum or platelet lysate.7,8 The International Society for Cellular Therapy has proposed more than 10 years ago minimal criteria for the definition of ex vivo-expanded MSCs based on their ability to adhere to plastic, expression of specific surface antigen expression (positivity for CD105, CD73, CD90; negativity for hematopoietic, endothelial and HLA class II markers) and capacity to differentiate in vitro into osteoblasts, chondroblasts, and adipocytes.9
Initially, the ready ability to expand and differentiate MSCs into several mesodermal lineages in vitro made these cells a promising tool for tissue engineering.10 More recently, several studies have demonstrated that MSCs display broad and potent immunoregulatory properties both in vitro and in vivo.11,12 In particular, MSCs are capable to sense inflammatory signals and adopt a pro- or anti-inflammatory phenotype to modulate innate and adaptive immunity.12 These properties have prompted their clinical use in the setting of hematopoietic stem cell transplantation (HSCT), especially for the treatment of immune-mediated complications.13 MSCs were first demonstrated to suppress in vitro T-lymphocyte proliferation induced by mitogens and alloantigens by releasing soluble factor such as indoleamine 2,3-dioxygenase (IDO) and PGE2.14 Thereafter, it was shown that their coculture in vitro with peripheral blood MNCs is associated with the differentiation of CD4+ T cells into CD25+FoxP3+ regulatory T cells15; this was confirmed in vivo in experimental models of autoimmune and inflammatory diseases.16 The MSC-driven polarization of T cells toward a Treg phenotype, together with their ability to interact with cells of the innate immune system, highlight the role of MSCs in the control of inflammation and maintenance of tissue homeostasis.12 Indeed, MSCs are capable to regulate the polarization of monocytes (M0) toward M1 and M2 macrophages after sensing the surrounding microenvironment, thus contributing to deliver both anti- and pro-inflammatory signals. In vitro, coculture of monocytes with BM-derived MSCs induces the differentiation of monocytes into M2 macrophages and their secretion of large amounts of the immunosuppressive and anti-inflammatory cytokine IL-10.17 MSC licencing in an inflammatory environment seems to be fundamental to mediate their immunoregulatory properties. For example, proinflammatory stimuli like IFNγ, TNF, or LPS increase the expression of IDO and the inhibitory effect of MSCs on T cells is more robust when MSCs are pretreated with proinflammatory factors, such as INFγ and TNFα.18
Thanks to their immunoregulatory and pleiotropic functions, MSCs has been employed as a therapeutic tool in the clinical arena both in the context of HSCT and in strategies of Regenerative Medicine. MSC treatment has been employed both to enhance hematopoietic stem cell (HSC) engraftment and to treat therapy-resistant acute graft-versus-host disease (aGvHD) after HSCT.13,19,20 In GvHD phase I/II studies, MSC intravenous infusion has been shown to be safe and a valuable therapy for patients with the severe, refractory aGvHD, especially in children and when performed early in the course of the disease.20,21 Phase III, multicenter, academic studies are ongoing in Europe with the aim to define the role of MSCs in the management of aGvHD and to identify product and patient's factors able to predict response to MSCs in vivo (HO113, EudraCT 2012-004915-30). In this respect, Galleu et al have recently reported that delivery of MSC immunosuppressive effect occurs after induction of perforin-dependent apoptosis by recipient cytotoxic T-cells and suggests that patients affected by GvHD should receive ex vivo apoptotic MSCs.22 MSCs have also been successfully employed in approaches of Regenerative Medicine to re-establish tissue homeostasis and promote repair in autoimmune and inflammatory disorders, such as refractory Crohn disease.23
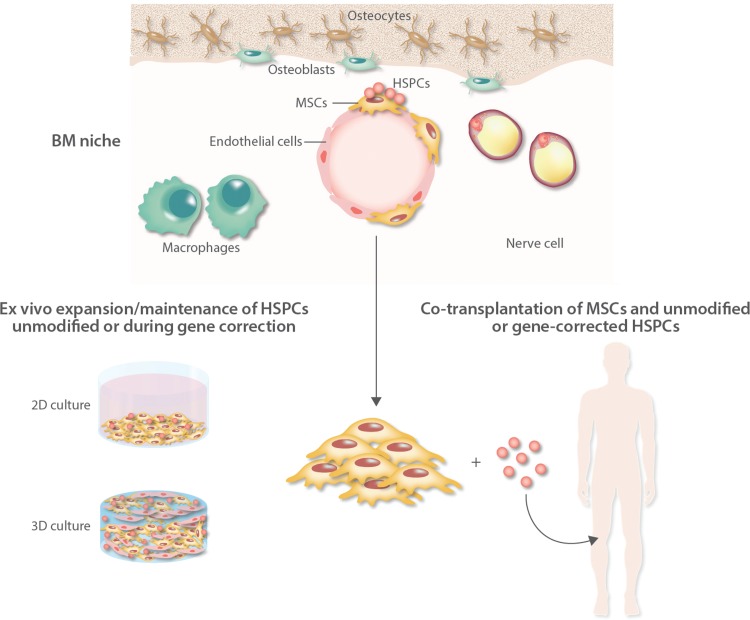
In this review, we critically discuss the role of MSCs in the maintenance of HSPCs within the BM niche, as well in transplantation strategies to facilitate HSC engraftment and expansion. Moreover, we will introduce their potential use in the context of ex vivo gene therapy with the aim to support both the expansion and the engraftment of gene-modified autologous HSCs (Fig. 1).
FIGURE 1.
Schematic representation of the bone marrow (BM) niche of the clinical applications of mesenchymal stromal cells to support engraftment of unmodified and gene-modified hematopoietic stem cells (HSCs). MSCs are crucial elements of the BM niche where, together with several nonhematopoietic cells including osteoblasts, adipocytes, endothelial, and neural cells, they provide newly formed osteoblasts for bone tissue regeneration and tightly control hematopoietic stem progenitor cell (HSPC) fate by direct interaction and through the secretion of soluble factors. MSCs have been employed in phase I/II clinical trials of hematopoietic stem cell transplantation (HSCT) to facilitate engraftment of HSPCs. Two different strategies are available: the ex vivo expansion and maintenance of primitive HSCs in 2D or 3D culture systems before infusion into patients (left side) and the coadministration of MSCs and HSCs in transplantation strategies (right side). These approaches can be applied not only in standard autologous and allogeneic transplantation with unmodified HSCs, but in the future also in ex vivo HSC-gene therapy especially when low numbers of gene-corrected cells are available (ie, after gene editing). MSC = mesenchymal stromal cells.
Role of MSCs in the BM niche
The understanding of the role of MSCs in the BM niche has been limited for a long time by the absence of in vivo MSC markers to track their phenotype and localization. However, the hypothesis that MSCs play a pivotal role in regulating the hematopoietic compartment in the BM niche was supported by data showing colocalization of MSCs with sites of hematopoiesis, starting from embryonic developmental stages.24
Identification of novel markers of mesenchymal progenitors and development of genetic tools to conditionally delete key factors in the niche has greatly increased in the recent years our knowledge on the mesenchymal compartment of the BM niche and on some of the pathways controlling its complex regulation. In particular, recent studies have been focused on identifying the MSC subsets which are mainly involved in the regulation of HSPC homeostasis.1–3
Sacchetti et al first reported that CD146 marker labels human BM cells residing in the sinusoidal wall that are enriched for CFU-F activity and are capable to support hematopoietic activity when transplanted as heterotopic ossicles in immunodeficient mice, by transferring a hematopoietic microenvironment.25 These cells could be isolated from the ossicles and were found to display clonogenic capacity and self-renewal ability in vivo. Mesenchymal cells localized in the trabecular region of the human BM that stained positive for CD271 marker were later identified and differentiated by the CD146+ perivascular cells. Also these CD271+ MSCs showed high CFU-F capacity, ability to transfer a BM microenvironment upon transplantation and to differentiate in vitro into mesodermal mature cell lineages.26 Altogether these data indicate that different subtypes of MSCs exist in the BM niche and interact with HSCs in specific perivascular regions. In particular, CD271+ and CD271+/CD146−/low MSCs have been described as bone-lining cells which are associated with long-term HSCs in low oxygen areas, whereas CD146+ and CD271+/CD146+ are located around BM sinusoids in association with activated and fast-proliferating HSPCs.26 However, a more dynamic concept of BM niche is emerging with primitive HSCs mainly localized along the sinusoidal vessels, closely associated with MSCs, integrating signals from the endosteal niche and able to migrate toward the bone-lining vessels, for example, after BM irradiation.27
One of the critical functions of MSCs within the niche is the secretion of soluble factors, including CXCL12 and stem cell factor (SCF), which contribute to HSPC maintenance. Nestin+ MSCs, which are innervated through parasympathetic nerve fibers, have been identified by Mendez-Ferrer group in mouse BM as niche key elements thanks to the expression of high levels of CXCL12. These cells display a perivascular location in close proximity with HSPCs and participate to the prenatal development of the HSC niche.28 Nestin+ cell subsets such as Nes-GFPbright and Nes-GFPdim cells were also identified: while the first is a rare population associated with arterioles, the second is more abundant and mainly accompanying sinusoids.29,30 Nes-GFPbright cells were later identified as NG2+ which have been reported to constitute an important source of CXCL12 factor, and not SCF, in the niche.30,31 The deletion of these perivascular Nestin-GFP+ cells using Nestin-Cre was associated with depletion of HSCs, highlighting their fundamental role in the niche as major producers of key factors.28
In a CXCL12-GFP knock-in mouse, CXCL12-abundant reticular cells were identified as a population of mesenchymal progenitors within the BM intratrabecular space that are capable to differentiate into osteoblasts and adipocytes in vitro and in vivo and to produce large amounts of SCF.32 The majority of these cells were found to express Leptin receptor (Lepr) and their depletion was paralleled by reduction in quiescent HSCs.32,33 Lepr expression has been employed to identify primitive MSCs in the adult murine BM and Lepr+ cells were found near the sinusoids and small diameter arterioles. Ding and Morrison demonstrated that the majority of MSCs positive for SCF or CXCL12 express also Lepr and that the deletion of these genes in the mesenchymal precursors by using a Lepr-driven Cre-recombinase was associated with depletion of HSCs.34
Mx-1 Cre-recombinase was found to identify a population of nonhematopoietic, nonendothelial mesenchymal progenitors in the bone which shows characteristics very similar to Nestin-GFP+ cells, including clonogenic capacity, ability to transfer hematopoietic activity and to undergo trilineage differentiation.35 Also paired related homeobox 1 (PRX1) and osterix (OSX) transcription factors have been reported to label mesenchymal progenitors in the BM with CFU-F activity and multilineage differentiation potential.36,37 Gremlin1 (Grem1) has been recently described to identify a population of mesenchymal precursors that does not express Nestin and displays the ability to differentiate into osteoblasts, chondrocytes, reticular marrow stromal cells, but not adipocytes in vivo.38
Available knowledge does not allow to distinguish between these different MSC populations based on their function because they all display clonogenic and differentiation capacities. Moreover, the anatomical localization of mesenchymal progenitors in relation with the niche vasculature has never been systematically analyzed.1–3 It is reasonable to speculate that MSCs are more frequently associated to sinusoids, given their higher number, as compared with arterioles. Noteworthy, it should be noted that artificial models based on the deletion of single molecules may not properly reflect the in vivo situation where a plethora of factors are produced by different niche cells that interact in a complex regulatory network to maintain HSPC functions.39 Therefore, a more detailed characterization of the MSC subsets in vivo, based on their physical localization, function and cytokine secretion profile, is necessary to better understand their biology and exploit them for clinical use.
MSCs in preclinical models of HSCT
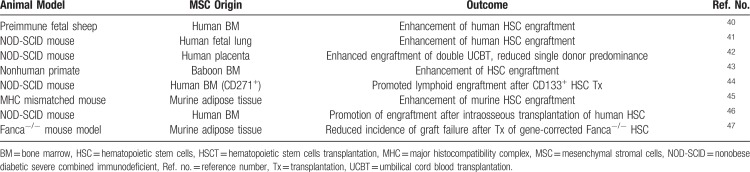
MSCs have been first demonstrated in experimental animal models to be capable to promote engraftment of HSCs and to prevent graft failure (see Table 1). Almeida-Porada et al40 observed that cotransplantation of human MSCs into preimmune fetal sheep resulted in enhancement of long-term engraftment of human cells in the BM and peripheral blood of the animals. Noort et al reported similar results in a cotransplantation model of fetal lung-derived MSCs and UCB-derived CD34+ cells in nonobese diabetic severe combined immunodeficient (NOD-SCID) mice and the enhanced engraftment could be maximized when low numbers of HSCs were coinfused with MSCs.41 In immunodeficient mice, cotransplantation of placenta-derived MSCs facilitated engraftment of double umbilical cord blood transplantation (UCBT) and reduced single cord predominance.42 Also in nonhuman primates, cotransplantation of baboon-derived MSCs improved HSC engraftment after intravenous infusion.43 Kuci et al reported that cotransplantation in NOD-SCID mice of CD133+ HSCs and CD271+ MSCs, one of the markers reported to label the most primitive and clonogenic MSCs, was associated with significantly greater lymphoid engraftment, as compared to the coinfusion of an unselected population of ex vivo-expanded MSCs.44 Fernández-García et al showed that the coinfusion of not only BM-derived, but also adipose tissue-derived MSCs with low numbers of HSCs significantly enhanced short- and long-term hematopoietic reconstitution in an autologous transplant setting in mice.45 Intraosseous cotransplantation of MSCs and HSCs could be also employed as a strategy to improve hematopoietic engraftment, as recently demonstrated in NSG mice human UCB-derived HSCs.46
Table 1.
MSCs in Preclinical Models of HSCT to Facilitate HSPC Engraftment
Altogether, these preclinical data confirm the ability of MSCs to support HSC engraftment in vivo in animal models of both autologous and allogeneic HSCT, in the absence of toxicity. This opens the possibility of employing MSCs also in the context of ex vivo HSC gene therapy where autologous, patient-derived HSCs are genetically modified ex vivo before infusion into patients. In this view, MSCs can be adopted in vitro as a tool to maintain primitive HSCs during the gene correction procedures and eventually employed in vivo to promote the engraftment of gene-corrected HSCs (see also Fig. 1 for a schematic representation). In this regard, Fernández-García et al have recently reported that the coinfusion of MSCs and gene-corrected autologous HSCs in a Fanconi anemia mouse model was associated with lower incidence of graft failure, especially when low numbers of gene-corrected Fanca−/− HSCs were infused.47 This strategy might be particularly useful in diseases in which a low number of corrected HSCs is available, such as for gene-edited HSCs, or the niche itself is involved in the pathophysiology of the disease and cannot properly support HSC engraftment, and also in case of reduced-intensity conditioning regimens.
MSCs in clinical trials of HSCT
Ex vivo-expanded MSCs have been administered to patients in a number of phase I/II clinical trials of HSCT with the aim of facilitating HSC engraftment (see Table 2) and to treat steroid-resistant aGvHD.13,19–21,48 While feasibility and safety of MSC infusions have been demonstrated in these studies, clinical efficacy needs be further proved in phase III randomized trials, together with the identification of product and patient's variables predicting response to MSC treatment.13,48
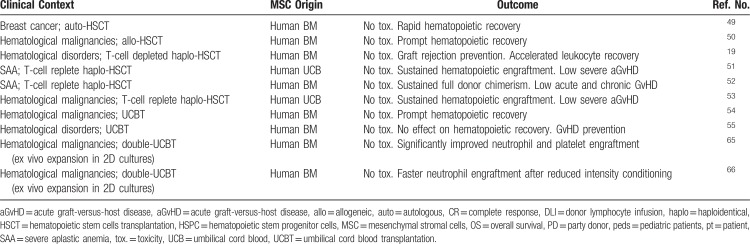
Table 2.
MSCs in Clinical Trials of HSCT to Facilitate HSC Engraftment
The first phase I/II clinical trial testing the coinfusion of MSCs in the context of HSCT enrolled 28 patients affected by breast cancer who received autologous peripheral blood mobilized HSCs and MSCs at a dose of 1 to 2 × 106 MSC/kg with the aim of accelerating hematological recovery. Patients showed rapid hematopoietic reconstitution in the absence of any toxicity.49 In another phase I/II, multicenter trial 46 patients undergoing allogeneic HSCT from an HLA-identical sibling for hematological malignancies received the coinfusion of MSCs; hematopoietic recovery was fast and no adverse reactions were registered.50 In the context of T-cell depleted HLA-haploidentical transplantation from a relative, 14 pediatric patients received within a Phase I/II study the coinfusion of MSCs at a standard dose of 1 to 2 × 106 MSC/kg and HSCs. No cases of either primary or secondary graft failure were registered in the study group, as compared with a 20% graft failure rate in the historical controls given HSCs only. Moreover, leukocyte recovery was faster in the MSC group as compared with the controls.19 Twenty-one pediatric and adult patients affected by severe aplastic anemia (SAA) and undergoing haplo-HSCT without T-cell depletion received the coinfusion of third-party donor UCB-MSCs with the double aim of facilitating HSC engraftment and preventing GvHD development. All patients showed sustained hematopoietic engraftment without any adverse reaction and a low incidence of severe aGvHD (5%).51 Similar results were reported in a larger group of 44 patients affected by SAA and undergoing non-T-cell depleted haplo-HSCT with the coinfusion of MSCs who showed a median time to neutrophil and platelet engraftment of 12 and 19 days, respectively, sustained full donor chimerism and a low incidence of grade II–IV acute and chronic GvHD.52 The coinfusion of third-party UCB-MSCs and haploidentical HSCs was also tested in 50 pediatric and adult patients affected by refractory/relapsed hematological malignancies where a sustained hematopoietic engraftment and low incidence of aGvHD was confirmed when MSCs were administered, in line with the other studies.53
In the setting of UCB transplantation, ex vivo-expanded MSCs and unrelated donor UCB HSPCs were administered to 8 children; also in this study safety record was good and neutrophil recovery was prompt (median time: 19 days) and sustained.54 In another phase I/II study, UCB-derived HSCs and parental MSCs were cotransplanted in 13 pediatric patients with no differences in the engraftment kinetics of UCB-HSCs between study patients and controls, while a protective effect on acute and chronic GvHD occurrence and no GvHD-associated transplant-related mortality were noted.55
Overall, these data demonstrate the feasibility and safety of cotransplanting HSCs and MSCs in the clinical setting; moreover, this approach was associated with prompt hematological recovery and low graft failure rate. A formal proof of the benefit of MSCs over standard HSCT (ie, w/o MSC coinfusion) should be obtained in randomized clinical trials.
The exact mechanisms by which MSCs exert their functions have not been completely clarified, although it is commonly believed that both cell-to-cell contact and secretion of soluble factors play a role.11,12 A potential mechanism through which MSCs might contribute to the engraftment of donor HSCs, thus reducing the risk of graft failure, is their ability to restore the BM microenvironment which has been damaged by the conditioning regimen through the secretion of paracrine mediators.12 This aspect may be particularly important in the context of genetic diseases affecting both the hematopoietic compartment and the BM niche,56,57 where the restoration of a proper environment is fundamental to facilitate the engraftment of gene-corrected HSCs. In the context of mismatched transplants such as haploidentical HSCT, MSCs may also be capable to modulate residual host alloreactivity thanks to their immunoregulatory properties which include cell-to-cell contact mechanisms and release of soluble factors.19 It is well known that MSCs release extracellular vesicles (EVs) that contain several classes of RNAs involved in the control of transcription, cell proliferation, and immune regulation. Recent studies have reported that the immune regulatory and anti-inflammatory signals delivered by MSCs may be mediated at least in part by MSC-derived EVs.58,59 A recent paper demonstrated the existence of an EV-mediated powerful cross-talk between MSCs and UCB-derived CD34+ cells capable to influence HSC biological functions, such as viability, differentiation, and migration.59 Moreover, MSC-EVs have been already employed in the clinical setting to alleviate symptoms of aGvHD in a patient with treatment resistant, grade IV manifestations likely due to reduction of the proinflammatory cytokine response, in the absence of side effects.60
Limited evidence is available also on the engraftment of human MSCs after infusion in vivo, which seems to occur for a very limited fraction of the cells, at least when ex vivo-expanded MSCs are employed.61 A recent paper from Abbuehl et al indicate that, after intrabone transplantation, primary, but not culture-expanded murine MSCs can engraft in the BM after irradiation.62 Another important limitation of our knowledge on MSC biology comes from the fact that experimental and clinical data available have been largely obtained with ex vivo cultured cells.11–13,19–22 Whether primary human MSCs in vivo display the same biological and functional properties of expanded cells is not clearly understood because of their low frequency in the BM which requires in vitro expansion.
In addition, MSCs have been shown to stimulate ex vivo expansion of HSCs before infusion into patients. In a 2D system, the coculture of UCB-derived HSCs and MSCs resulted in higher numbers of total nucleated cells, CD34+ and CD133+ HSCs and CFU-F activity, as compared with standard liquid culture.63,64 In a phase I/II clinical trial of double UCBT, 2 UCB units, 1 expanded ex vivo over MSCs and 1 unexpanded, were infused into 31 adult patients with hematological malignancies. Significantly improved neutrophil and platelet engraftment, as compared with historical controls receiving 2 unmanipulated UCB units, was registered together with a good safety profile of the approach.65 The same group recently confirmed these results in 27 patients undergoing double UCBT after a reduced intensity conditioning regimen and reported a cumulative incidence of neutrophil engraftment on day 26 of 75% with UCB expansion versus 50% without expansion.66
The demonstration that the human niche can be modeled in vitro by MSC-HSC coculture has been widely reported; however, the spatial relationship between these 2 cell types in a 2D culture system during ex vivo expansion has been investigated by Jing et al.67 The authors identified 3 distinct localizations of HSPCs in vitro in relation to the stromal layer: cells floating in the supernatant, phase-bright cells adhering to the MSC surface and containing the highest proportion of proliferating progenitors and phase-dim cells localized beneath the MSC layer cells and characterized by a lower cycling rate and a more immature phenotype. Thanks to these hematopoietic supportive properties, MSCs could also be adopted to maintain and expand ex vivo primitive HSCs during gene correction procedures in gene therapy approaches. Preliminary data from our group support the finding that MSCs are capable not only to expand HSCs in 2D cultures, but also to preserve their primitive phenotype (Crippa et al, unpublished) defined according to specific surface markers able to identify primitive HSC subsets among CD34+ cells.68 Moreover, 3D scaffold models based on bioreactors are now available to reproduce ex vivo the BM niche and to sustain more efficiently primitive HSCs.69 Another possible approach could be the selection of a specific MSC subset to improve the hematopoietic supportive capacity of MSCs in vitro and in vivo.44 Finally, humanized ossicle models which allow to mimic in vivo in NSG mice a humanized BM niche are becoming available70; these models will greatly improve our understanding of the biology and architecture of the mesenchymal compartment, as well as of their interactions with HSPCs in healthy subjects and in patients (Fig. 1 for a schematic representation).
Conclusions and future directions
Clear evidence from preclinical and clinical data indicates that MSCs are key elements in the BM niche where they participate to the regulation of HSPC homeostasis and fate through complex regulatory networks.1–3 The use of putative markers for the prospective isolation of primary MSCs in vivo and of genetically modified mice with conditional deletion of key niche factors have significantly improved our understanding of MSC1–3; however, further studies are needed to augment our knowledge on the biology of the different MSC subsets and their hierarchical organization within the BM niche. In vivo models, in which a single factor in a specific mesenchymal cell subset is deleted, have clear limitations and may not reflect the in vivo situation and the relationship between different MSC subpopulations endowed with different functions.39 A thorough comparison between MSC subsets in vivo and their ex vivo-expanded counterparts in terms of immunophenotype, functional abilities, and secretome profile is also needed to better instruct the clinical application of the cells.
Clinical data from phase I/II studies support the clinical use of MSCs in the setting of autologous and allogeneic HSCT with the aim to facilitate engraftment of HSCs. Two different strategies can be considered: the ex vivo expansion and maintenance of primitive HSCs in 2D or 3D culture systems64,65,69 before infusion into patients and the coadministration of MSCs and HSCs in transplantation strategies (Fig. 1).19,49,50 These approaches could be employed in the future not only in the setting of standard autologous and allogeneic transplantation, but also in ex vivo HSC-gene therapy approaches to optimize its outcome when low numbers of corrected cells are available (ie, after gene editing).71
Footnotes
Citation: Crippa S, Bernardo ME. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoieitic Stem Cell Transplantation. HemaSphere, 2018;2:6. http://dx.doi.org/10.1097/HS9.0000000000000151.
Funding/support: This work has been supported by grants from the Fondazione Telethon to Maria Ester Bernardo.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity 2018; 48:632–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015; 16:239–253. [DOI] [PubMed] [Google Scholar]
- 3.Asada N, Takeishi S, Frenette PS. Complexity of the bone marrow hematopoietic stem cell niche. Int J Hematol 2017; 106:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968; 6:230–247. [PubMed] [Google Scholar]
- 5.Li H, Ghazanfari R, Zacharaki D, et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci 2016; 1370:109–118. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo ME, Cometa AM, Pagliara D, et al. Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol 2011; 24:73–81. [DOI] [PubMed] [Google Scholar]
- 7.Avanzini MA, Bernardo ME, Cometa AM, et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypical and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica 2009; 94:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingo DM, Redaelli D, Rossella V, et al. Bone marrow-derived CD34- fraction: a rich source of mesenchymal stromal cells for clinical application. Cytotherapy 2016; 18:1560–1563. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315–317. [DOI] [PubMed] [Google Scholar]
- 10.Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int 2017; 2017:5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13:392–402. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo ME, Fibbe WE. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol Lett 2015; 168:215–221. [DOI] [PubMed] [Google Scholar]
- 14.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indolamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103:4619–4621. [DOI] [PubMed] [Google Scholar]
- 15.Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 2013; 91:12–18. [DOI] [PubMed] [Google Scholar]
- 16.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009; 15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melief SM, Geutskens SB, Fibbe WE, et al. Multipotent stromal cells skew monocytes towards an anti-inflammatory IL-10 producing phenotype by production of IL-6. Haematologica 2013; 98:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.François M, Romieu-Mourez R, Li M, et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 2012; 20:187–195. [DOI] [PubMed] [Google Scholar]
- 19.Ball LM, Bernardo ME, Roelofs H, et al. Co-transplantation of ex-vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem cell transplantation. Blood 2007; 110:2764–2767. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 21.Ball LM, Bernardo ME, Roelofs H, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol 2013; 163:501–509. [DOI] [PubMed] [Google Scholar]
- 22.Galleu A, Riffo-Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 2017; 9:416. [DOI] [PubMed] [Google Scholar]
- 23.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut 2011; 60:788–798. [DOI] [PubMed] [Google Scholar]
- 24.Mendes SC, Robin C, Dzierzak E. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development 2005; 132:1127–1136. [DOI] [PubMed] [Google Scholar]
- 25.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007; 131:324–336. [DOI] [PubMed] [Google Scholar]
- 26.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 2011; 117:5067–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006; 124:407–421. [DOI] [PubMed] [Google Scholar]
- 30.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013; 502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asada N, Kunisaki Y, Pierce H, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 2017; 19:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010; 33:387–399. [DOI] [PubMed] [Google Scholar]
- 33.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014; 15:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 2012; 10:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 2014; 29:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015; 160:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beerman I, Luis TC, Singbrant S, et al. The evolving view of the hematopoietic stem cell niche. Exp Hematol 2017; 50:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida-Porada G, Porada CD, Tran N, et al. Co-transplantation of human stromal cell progenitors into pre-immune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood 2000; 95:3620–3627. [PubMed] [Google Scholar]
- 41.Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Exp Hematol 2002; 30:870–878. [DOI] [PubMed] [Google Scholar]
- 42.Hiwase SD, Dyson PG, To LB, et al. Cotransplantation of placental mesenchymal stromal cells enhances single and double cordblood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem Cells 2009; 27:2293–2300. [DOI] [PubMed] [Google Scholar]
- 43.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001; 29:244–255. [DOI] [PubMed] [Google Scholar]
- 44.Kuci S, Kuci Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 2010; 95:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-García M, Yañez RM, Sánchez-Domínguez R, et al. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther 2015; 6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metheny L, III, Eid S, Lingas K, et al. Intra-osseous co-transplantation of CD34-selected umbilical cord blood and mesenchymal stromal cells. Hematol Med Oncol 2016; 1:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-García M, Luisa Lamana M, Hernando-Rodríguez M, et al. Improved hematopoietic gene therapy in a mouse model of Fanconi anemia mediated by mesenchymal stromal cells. Hum Gene Ther 2018; 29:327–336. [DOI] [PubMed] [Google Scholar]
- 48.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 2018; 22:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after co-infusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18:307–316. [DOI] [PubMed] [Google Scholar]
- 50.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11:389–398. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Cao Y, Li X, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res 2014; 12:132–138. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Zhang Y, Xiao H, et al. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transplant 2017; 52:704–710. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Wang Z, Cao Y, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol 2013; 92:1675–1684. [DOI] [PubMed] [Google Scholar]
- 54.MacMillan ML, Blazar BR, DeFor TE, et al. Transplantation of culture-expanded haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant 2008; 43:1–8. [DOI] [PubMed] [Google Scholar]
- 55.Bernardo ME, Ball LM, Cometa AM, et al. Co-infusion of ex vivo expanded, parental mesenchymal stromal cells prevents life-threatening acute GvHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant 2011; 46:200–207. [DOI] [PubMed] [Google Scholar]
- 56.Starc N, Ingo D, Conforti A, et al. Biological and functional characterization of bone marrow-derived mesenchymal stromal cells from patients affected by primary immunodeficiency. Sci Rep 2017; 7:8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantelli M, Avanzini MA, Rosti V, et al. Comprehensive characterization of mesenchymal stromal cells from patients with Fanconi anaemia. Br J Haematol 2015; 170:826–836. [DOI] [PubMed] [Google Scholar]
- 58.De Luca L, Trino S, Laurenzana I, et al. Mesenchymal stem cell derived extracellular vesicles: a role in hematopoietic transplantation? Int J Mol Sci 2017; 18:E1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Luca L, Trino S, Laurenzana I, et al. miRNAs and piRNAs from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: a new insight in transplantation. Oncotarget 2016; 7:6676–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014; 28:970–973. [DOI] [PubMed] [Google Scholar]
- 61.von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012; 30:1575–1578. [DOI] [PubMed] [Google Scholar]
- 62.Abbuehl JP, Tatarova Z, Held W, et al. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell 2017; 21:241–255. [DOI] [PubMed] [Google Scholar]
- 63.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant 2006; 37:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadekar D, Kale V, Limaye L. Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34(+) cells. Stem Cell Res Ther 2015; 6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012; 367:2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehta RS, Saliba RM, Cao K, et al. Ex vivo mesenchymal precursor cell-expanded cord blood transplantation after reduced-intensity conditioning regimens improves time to neutrophil recovery. Biol Blood Marrow Transplant 2017; 23:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jing D, Fonseca AV, Alakel N, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells-modeling the niche compartments in vitro. Haematologica 2010; 95:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basso-Ricci L, Scala S, Milani R, et al. Multiparametric whole blood dissection: a one-shot comprehensive picture of the human hematopoietic system. Cytometry A 2017; 91:952–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fritsch K, Pigeot S, Feng X, et al. Engineered humanized bone organs maintain human hematopoiesis in vivo. Exp Hematol 2018; 61:45–51. [DOI] [PubMed] [Google Scholar]
- 70.Abarrategi A, Foster K, Hamilton A, et al. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J Clin Invest 2017; 127:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 2014; 510:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]