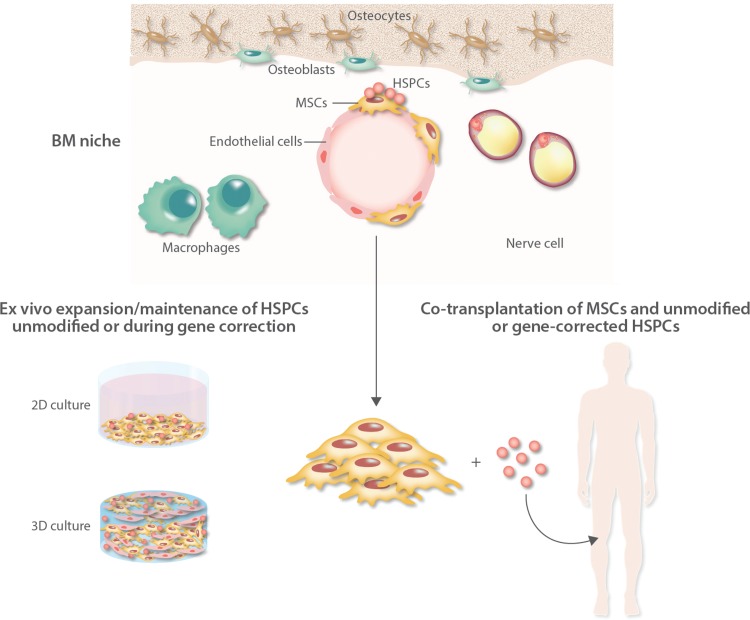
FIGURE 1.
Schematic representation of the bone marrow (BM) niche of the clinical applications of mesenchymal stromal cells to support engraftment of unmodified and gene-modified hematopoietic stem cells (HSCs). MSCs are crucial elements of the BM niche where, together with several nonhematopoietic cells including osteoblasts, adipocytes, endothelial, and neural cells, they provide newly formed osteoblasts for bone tissue regeneration and tightly control hematopoietic stem progenitor cell (HSPC) fate by direct interaction and through the secretion of soluble factors. MSCs have been employed in phase I/II clinical trials of hematopoietic stem cell transplantation (HSCT) to facilitate engraftment of HSPCs. Two different strategies are available: the ex vivo expansion and maintenance of primitive HSCs in 2D or 3D culture systems before infusion into patients (left side) and the coadministration of MSCs and HSCs in transplantation strategies (right side). These approaches can be applied not only in standard autologous and allogeneic transplantation with unmodified HSCs, but in the future also in ex vivo HSC-gene therapy especially when low numbers of gene-corrected cells are available (ie, after gene editing). MSC = mesenchymal stromal cells.