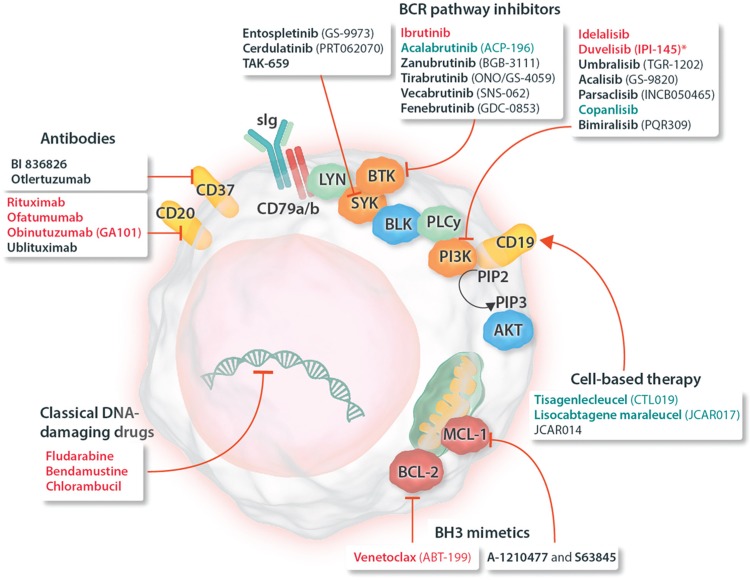
Figure 1.
Schematic representation of a CLL cell with established and experimental drug targets, as well as a classification of respective drugs (approved and experimental). Names of drugs with approval for use in CLL are given in red; drugs approved for use in other indications are shown in blue; drugs in various stages of clinical development are shown in black. ∗Duvelisib has been approved for treatment of CLL by the FDA but not yet by the EMA. AKT = protein kinase B, BCL-2 = B-cell lymphoma 2, BCL-XL = B-cell lymphoma-extra large, BCR = B-cell receptor, BLK = B lymphocyte kinase, BTK = Bruton tyrosine kinase, CLL = chronic lymphocytic leukemia, EMA = European Medicines Agency, FDA = Food and Drug Administration, LYN = LCK/YES novel tyrosine kinase, MCL-1 = induced myeloid leukemia cell differentiation protein Mcl-1, PD-1 = programmed cell death protein 1, PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase, PIP2 = phosphatidylinositol (4,5)-bisphosphate, PIP3 = phosphatidylinositol (3,4,5)-trisphosphate, PLC = phospholipase C, sIg = surface immunoglobulin, SYK = spleen tyrosine kinase.