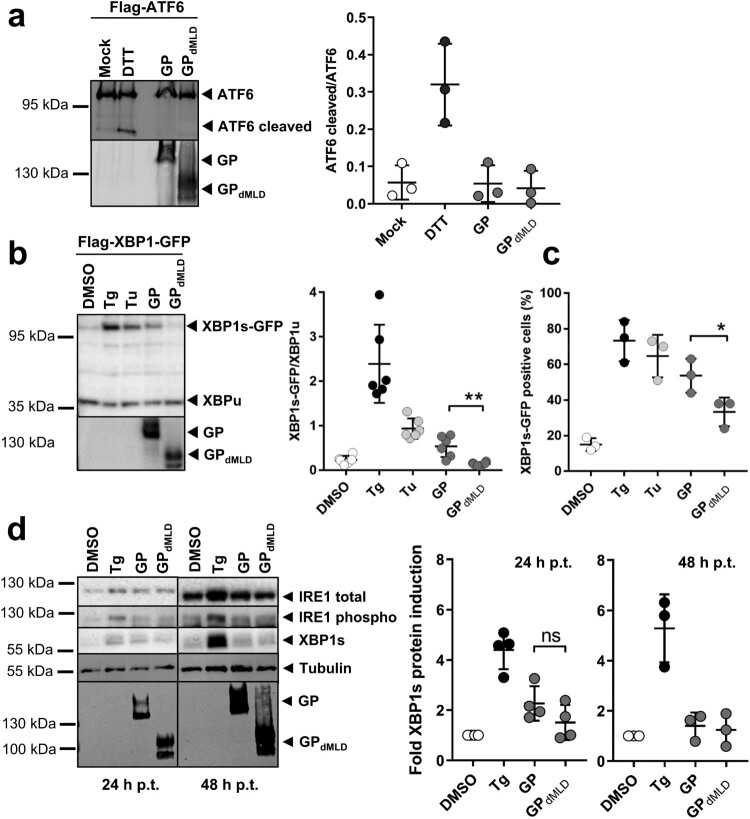
Figure 2.
MARV GP activates UPR in an IRE1/XBP1-dependent manner. (a) HuH7 cells transfected with plasmids encoding Flag-ATF6 and GP or GPdMLD (1 µg each) were lysed 48 h p.t. and analysed by Western blotting using an anti-Flag mouse monoclonal antibody and an Alexa680-conjugated anti-mouse antibody to detect full-length and cleaved (active) ATF6. MARV-specific goat serum and an IRdye800-conjugated anti-goat antibody were used to detect the viral proteins. Incubation of cells with 1 mM DTT for 30 min served as a positive control. Detection and quantification were performed using an Odyssey imaging system. The ratio of cleaved ATF6 protein to full-length ATF6 protein was calculated. The experiment was performed three times. (b) HuH7 cells were transfected with plasmids encoding Flag-XBP1-GFP, GP (1 µg), GPdMLD (25 ng) or empty vector (DMSO, Tg, Tu). The total amount of transfected plasmid (2 µg in total) was kept constant by the addition of empty vector. XBP1 splicing was induced by 5 nM Tg or 300 nM Tu for 16 h. The cells were lysed at 48 h p.t. and analysed by Western blotting using monoclonal antibodies against the Flag-tag and GP and peroxidase-coupled secondary antibodies. XBP1s and XBP1u were quantified using the ChemiDoc imaging system, and the ratios of these proteins were calculated. The experiment was performed six times. (c) HuH7 cells that had been treated and transfected as explained in b were fixed 48 h p.t. and subjected to immunofluorescence analysis. DMSO, Tg and Tu: HuH7 cells were transfected with an mCherry-expressing plasmid instead of with empty vector and were treated as indicated in b. Viral proteins were stained using monoclonal protein-specific and fluorescently labelled secondary antibodies. XBP1s-GFP positive nuclei were counted in cells expressing the viral protein or mCherry in three independent experiments. The percentage of XBP1s-GFP positive nuclei is shown. Each circle represents the result from an individual experiment, data are shown as the means ± SD. (d) HuH7 cells were transfected with plasmids encoding GP (1 µg), GPdMLD (200 ng) or mCherry (DMSO, Tg). The total amount of transfected plasmid (2 µg in total) was kept constant by the addition of mCherry plasmid. Cells were lysed at 24 and 48 h p.t. and subjected to Western blot analysis to detect endogenous IRE1 and XBP1s proteins using protein-specific antibodies detected by POD-coupled secondary antibodies. 24 and 48 h samples were analysed in parallel on the same blot afterwards tubulin and MARV GP were detected. XBP1s levels were quantified and presented as relative values to DMSO-treated cells (set to 1). The experiments were performed four (24 h) or three (48 h) times. Each circle represents a sample from an individual experiment, data are shown as the means ± SD.