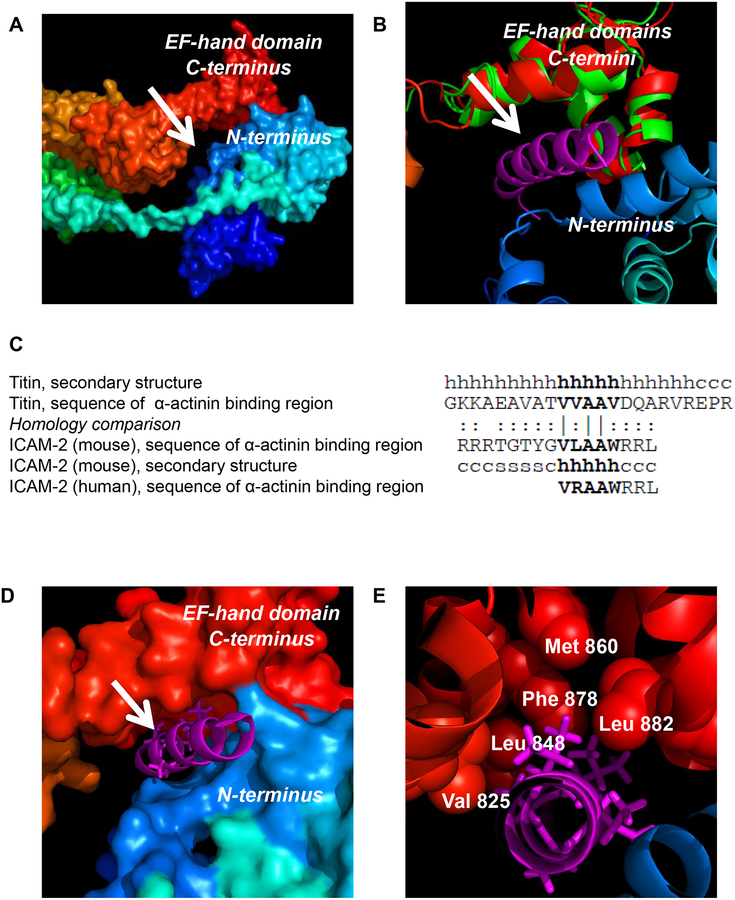
Figure 2. Modeling of ICAM-2 interaction with α-actinin.
(A) Surface representation of the full-length chicken α-actinin structure (PDB code 1SJJ) reveals a potential binding cavity (arrow) in the cleft between the actin binding domain (ABD, blue) and EF-hand (red) domains. The N-terminus to C-terminus is colored from blue (ABD) to red (EF-hand domain). (B) Structural alignment between full-length chicken α-actinin (red) and the EF-hand domain of human α-actinin (green)(PDB code 1H8B) demonstrated a high confidence alignment (RMSD of 1.47 Å). Structural alignment of the EF-hand domain of chicken α-actinin (red) with EF-hand domain of human α-actinin (residues 823–894, green) complexed to the α-actinin binding domain helix of rabbit titin (known structure, magenta) placed this helix in the putative binding pocket suggested by the structure in “A”. (C) The sequences of the α-actinin binding domains of rabbit titin, murine ICAM-2, and human ICAM-2 have high homology. The secondary structure of the rabbit and murine peptides are indicated as: “h” = helix, “s” = β-sheet and “c” = coil. Amino acid homology comparison is indicated as: “|” = identical; “:” = similar. (D) Modeling reveals a likely high-affinity interaction between the structurally conserved alpha-helix of titin (magenta) and the cytoplasmic domains of murine and human ICAM-2 (by structural and sequence homology) with the EF-hand domain of α-actinin (red). (E) Specific hydrophobic residues of the EF-hand domain of α-actinin and adjacent hydrophobic residues of a helical α-actinin binding structure suggest that ICAM-2 associates directly with α-actinin through hydrophobic interactions. The magenta stick structures represent the “VVAAV” component of the titin helix that is most conserved in ICAM-2.