Fig 1. The overall architecture of Can f 6 displays the classic lipocalin structure.
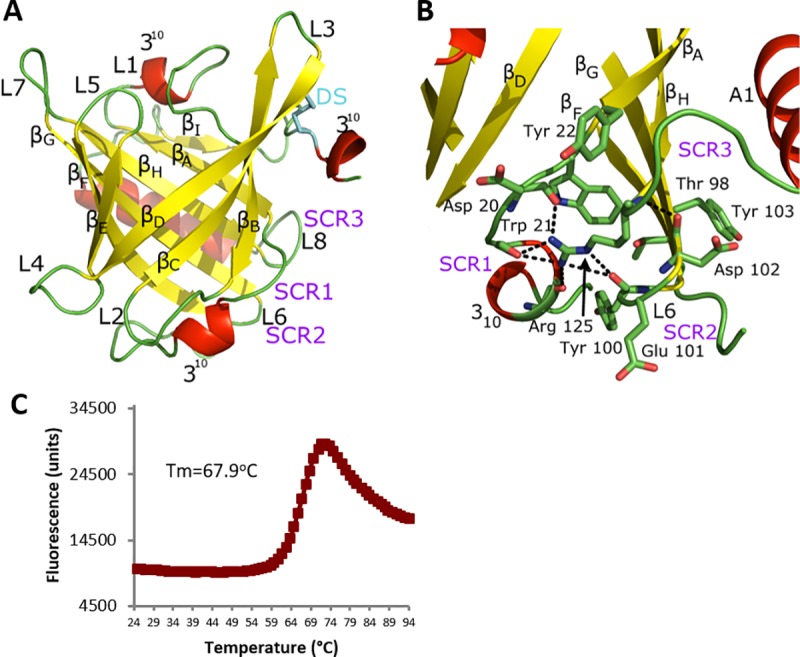
(A) Eight antiparallel β-strands (yellow) form a central ligand binding cavity (βA-I) flanked by C-terminal α-helix (posteriorly located in the figure) and three 310-helices (red), with the connecting loops (L, in green) numbered. A disulfide bond (DS, cyan) clamps the C-terminal loop to the beta core via βD. The cavity opening is covered by L1 and a loop-310 helix-loop that links together βA and βD. Three core lipocalin Structurally Conserved Regions (SCR, purple) that hold the overall fold together are labeled: SCR1 connects the N-terminal loop to the C-terminal SCR3 which also interacts with SCR2. (B) Figure A is rotated by 90o out of the plane and shows the main residues of the core SCR. In SCR3, the side chain and distal guanidium group of a conserved Arg125 are stabilized by hydrogen bonds (dashed lines) to the backbone carbonyls of other SCR residues and a Cation-Pi interaction with a conserved SCR1 aromatic residue, here Trp21. Other residues in the SCR also form numerous non-covalent interactions. (C) Purified Can f 6 was assayed by Thermofluor shift to demonstrate the stability of our recombinant Can f 6. The average Tm is 67.9°C with standard deviation of 1.7°C.
