Figure 5.
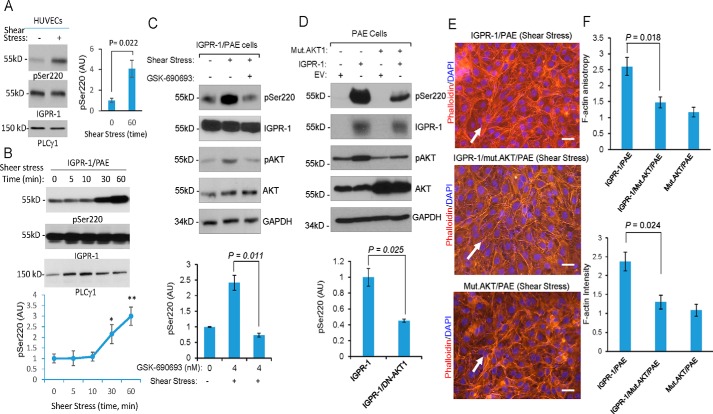
Shear stress–dependent phosphorylation of AKT mediates phosphorylation of IGPR-1. A, fully confluent HUVECs were kept in either normal static or under shear stress conditions. After 60 min, the cells were lysed, and whole-cell lysates were blotted for pSer-220, total IGPR-1, and PLCγ1. Shown is the normalized phosphorylation of Ser-220 to total IGPR-1, which is representative of three independent experiments. B, fully confluent IGPR-1/PAE cells were kept in normal static or under shear stress conditions for various times as indicated. The cells were lysed, and whole-cell lysates were blotted for pSer-220, total IGPR-1, and PLCγ1. The graph is representative of three independent experiments. Phosphorylation of Ser-220 was normalized to total IGPR-1. C, IGPR-1/PAE cells were incubated under shear stress in the presence of control vehicle or the pan-AKT inhibitor GSK-690693. The cells were lysed, and whole-cell lysates were blotted for pSer-220, IGPR-1, p-AKT, total AKT, and GAPDH. The graph is representative of three independent experiments. D, fully confluent PAE cells expressing EV, IGPR-1, mutant AKT1 (K179M/T308A/S473A) alone or co-expressed with IGPR-1 and mutant AKT1 were placed under shear stress for 6 h. The cells were lysed, and whole-cell lysates were blotted for pSer-220 (pSer220), total IGPR-1, AKT, and GAPDH. The graph is representative of three independent experiments. E, the fully confluent cell lines under static or shear stress were fixed and stained with phalloidin for actin (red) and DAPI (blue). F, F-actin orientation (anisotropy) and expression were quantified using an open source plugin Fibriltool. Scale bar, 10 μm. Mut., mutant.