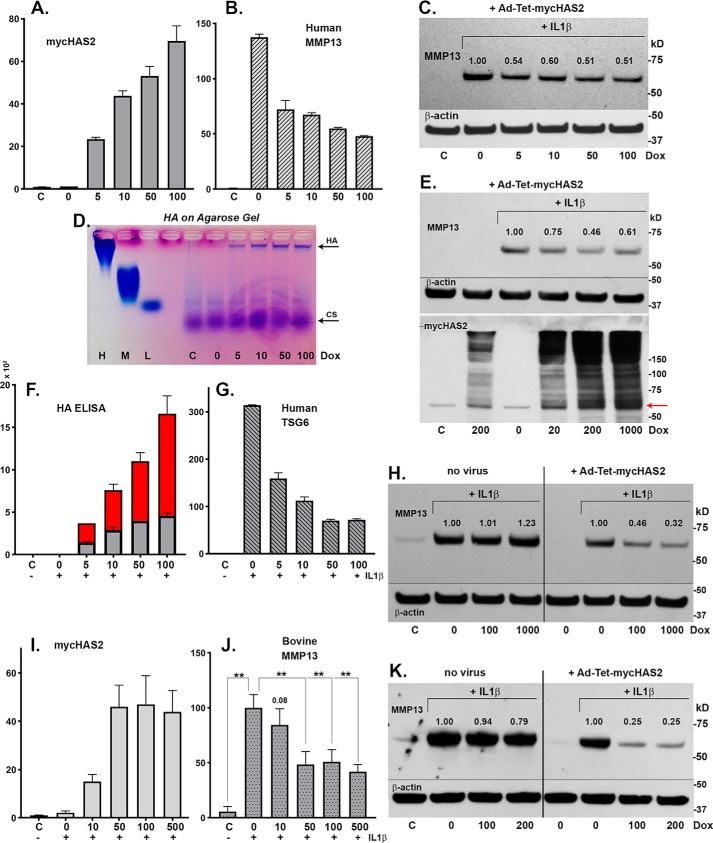
Figure 3.
Human chondrocytes were transduced with 100 IFU/cell Ad-Tet-mycHAS2; untreated control is labeled C. Following transduction, the chondrocytes were treated with 1 ng/ml IL1β (bars 2–6 or labeled with a plus sign), exposed to varying Dox concentrations (as labeled, ng/ml) and analyzed for changes in mycHAS2 (A), MMP13 (B), and TSG6 mRNA (G). Chondrocyte lysates were analyzed by Western blot analysis of MMP13 protein (C; blots reprobed for β-actin). Media from these cultures were analyzed on an agarose electrophoresis sizing gel (D) and by HA ELISA (F; red, medium; gray, cell-associated HA in units of ng/ml × 103). Western blot analyses were performed on three additional human OA chondrocyte cultures (E, H, and K) exposed to Dox (as labeled, ng/ml). Blots were probed for MMP13 protein, β-actin, or mycHAS2 as labeled. Numbers shown above MMP13 bands indicate band intensity (normalized to β-actin) relative to the IL1β-induced MMP13 band set to 1.0. H and K, two experiments in which nontransduced (no virus as labeled) and Ad-Tet-mycHAS2–transduced human OA chondrocytes were exposed to Dox (as labeled, ng/ml). I and J, the effect of Dox concentrations on transduced bovine chondrocytes without (C) or with 1 ng/ml IL1β (+) on expression for mycHAS2 (I) or MMP13 (J) mRNA; results from independent bovine cultures n = 3 for each condition (n = 12 for 100 ng/ml Dox comparison). I, -fold change relative to control set to 1.0; J, -fold change relative to values with IL1β treatment (without Dox) set to 100. An unpaired t test was used for statistical analysis: **, p < 0.01. Error bars, S.D.