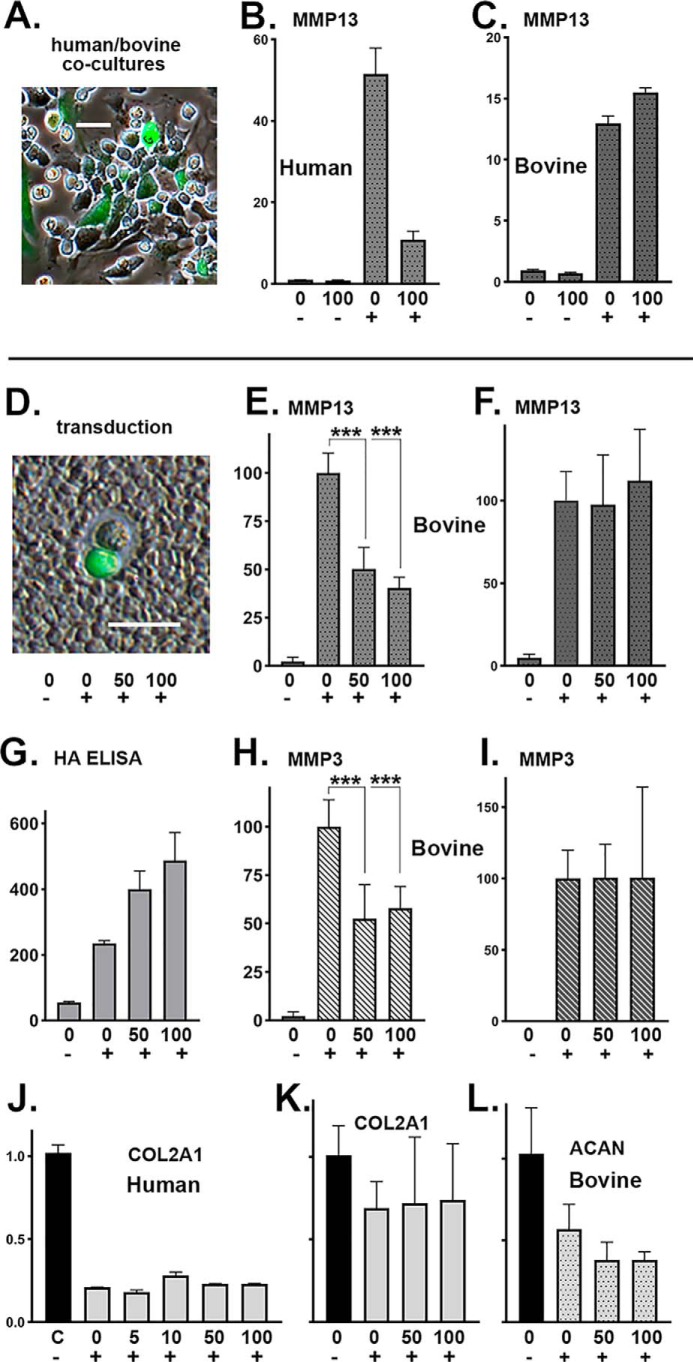
Figure 6.
Ad-Tet-mycHAS2–transduced human chondrocytes were co-cultured with nontransduced bovine chondrocytes. A, example of a coculture, except Ad-ZsGreen-mycHAS2 was used. The co-cultures (B and C) were treated for 24 h with 0 or 100 ng/ml Dox (as labeled) and without (−) or with (+) 1 ng/ml IL1β. B and C, qRT-PCR using human-specific (B) or bovine-specific (C) primers for MMP13. Data represent duplicate experiments and depict relative -fold change with control lysates set to 1.0. In a second series of experiments (E–L), bovine chondrocytes were transduced with Ad-Tet-mycHAS2, wherein >50% were successfully transduced (D; coat image using Ad-ZsGreen-mycHAS2 for illustration). Transduced chondrocyte cultures were incubated with 0, 50, or 100 ng/ml Dox (as labeled) and without (−) or with (+) 1 ng/ml IL1β. After 24 h of treatment, the conditioned medium from each of these cultures was added to fresh, nontransduced bovine chondrocytes (F and I) and allowed to incubate for an additional 24 h. The conditioned medium was also analyzed for HA content via an HA ELISA (G; units of ng of HA/ml). Analysis by qRT-PCR used bovine-specific primers for MMP13 (E and F), MMP3 (H and I), COL2A1 (K), and ACAN (L). The -fold changes in bovine mRNA are relative to values with IL1β treatment (without Dox) set to 100. E–I, results from six independent bovine cultures for each condition. An unpaired t test was used for statistical analysis; ***, p < 0.001. J, -fold change expression of human COL2A1 mRNA using lysates of the human OA chondrocyte experiment shown in Fig. 3B. Error bars, S.D.