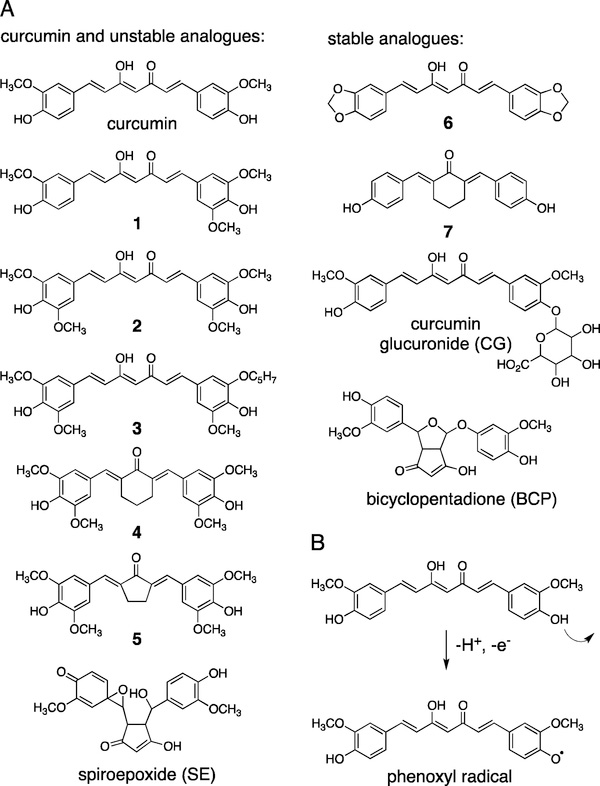
Fig. 1.
(A) The chemical structures of curcumin, synthetic analogues, and metabolites used in this study. Compounds are grouped into chemically unstable and stable depending on their propensity to undergo spontaneous degradation in buffer (15,18). (B) Degradation of curcumin is initiated by loss of a hydrogen from the phenolic hydroxyl group. The resulting phenoxyl radical undergoes cyclization and oxygenation reactions that result in formation of a spiroepoxide intermediate en route to bicyclopentadione as the major stable degradation product (16).