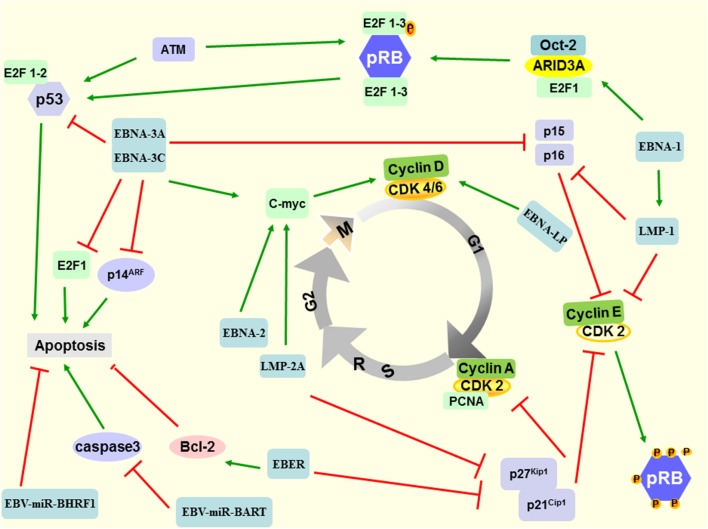
Fig. 3.
A schematic diagram of EB virus involved in the G1/S transition. Epstein–Barr virus infection is an early event in the development of malignancies. The latent proteins and miRNAs encoded by EBV in host cells alone or in combination drive the cell cycle through a variety of pathways. LMP-1 regulates telomerase activity through the p16INK4A/Rb/E2F1 signaling pathway to promote cell immortalization. LMP-2A couples with c-Myc to promote G1/S transition and hyperproliferation of B lymphocytes through promoting the expression of cyclin D and the degradation of p27kip1 at the early stage of oncogenesis. The cell cycle regulatory protein E2F1, the E2F-binding protein ARID3A, and the B-cell-specific transcription factor Oct-2 bind EBNA-1, which are necessary for transcriptional activation. EBNA-1 also enhances expression of LMP-1, and then promotes cell proliferation. The interaction between EBNA-2, EBNA-3C, and c-Myc further activates cyclin D2 and CDK4, then promoting the cell from G1 phase into S phase. EBNA-3A and EBNA-3C down-regulate the expression of p15INK4b, p16INK4a, and p14ARF, thereby inhibiting apoptosis. EBAN-3C can directly bind to p53, to a certain extent, inhibit its transcriptional activity. EBERs can up-regulate Bcl-2 and down-regulate p21cip1 and p27kip1, thereby releasing the inhibition of CDK4 and CDK2 and promoting the cell cycle from G1 phase to S phase. EBV-miR-BHRF1 inhibits apoptosis in B lymphocytes and epithelial cells. EBV-miR-BARTs can target caspase 3, thereby inhibiting apoptosis and increasing the number of cells entering the S phase