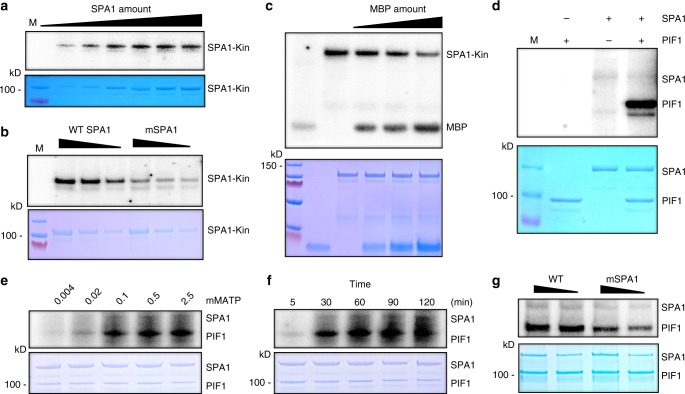
Fig. 1.
SPA1 acts as a ser/thr protein kinase. a SPA1 kinase domain (SPA1-Kin) purified from E. coli showed an auto-phosphorylation activity in a concentration-dependent manner (autoradiogram on top panel). Bottom panel shows the protein level in a Coomassie-stained gel. M, indicates protein marker. b A conserved amino acid mutation on the SPA1 kinase domain reduces the auto-phosphorylation activity of SPA1 (autoradiogram on top panel). Bottom panel shows the protein level in a Coomassie-stained gel. c The N-terminal kinase domain of SPA1 exhibits kinase activity in the presence of myelin basic protein (MBP); a general kinase substrate (autoradiogram on top panel). Bottom panel shows the protein levels in a Coomassie-stained gel. d Full-length SPA1 purified from Pichia pastoris phosphorylates PIF1 in vitro (autoradiogram on top panel). Bottom panel shows the protein levels in a Coomassie-stained gel. e ATP-dependent kinase assays of full-length SPA1 on PIF1 (autoradiogram on top panel). ATP concentrations used were 0.004, 0.02, 0.1, 0.5, and 2.5 mM. Bottom panel shows the protein levels in a Coomassie-stained gel. f Kinetic analysis of the kinase activity of the full-length SPA1 on PIF1 (autoradiogram on top panel). Bottom panel shows the protein levels in a Coomassie-stained gel. g A conserved amino acid mutation on the full-length SPA1 kinase domain reduces the SPA1 kinase activity toward PIF1 (autoradiogram on top panel). Bottom panel shows the protein levels in a Coomassie-stained gel