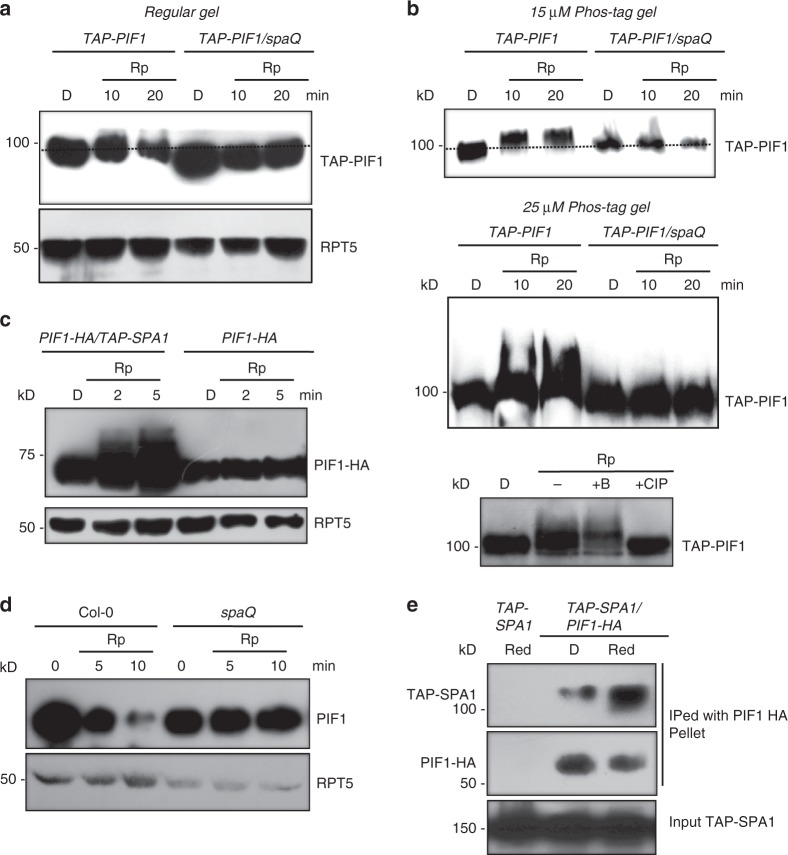
Fig. 2.
SPAs are necessary for the light-induced phosphorylation and degradation of PIF1 in vivo. a Immunoblots showing the light-induced phosphorylation of TAP-PIF1 is defective in the spaQ mutant compared to wild type. Four-day-old dark-grown seedlings were either kept in darkness or exposed to a pulse of red light (300 μmolm−2) and then incubated in the dark for the duration indicated before being sampled for protein extraction. All dash lines show the dark position of the PIF1 band. b Immunoblots show a defect in the light-induced phosphorylation of TAP-PIF1 in spaQ background compared to wild type in gels containing 15 (top) and 25 (middle) µM phostag. (Bottom panel) The red light-induced slow-migrating band is a phosphorylated form of TAP-PIF1 as indicated by the phosphatase treatment. +CIP, native Calf Intestinal Phosphatase; +B, heat-inactivated boiled CIP. c Overexpression of SPA1 induces faster phosphorylation of PIF1-HA than wild type under weak red-light pulse (1 μmolm−2). Seedlings were pretreated with the proteasome inhibitor (40 μM bortezomib) for 5 h before being exposed to red light. Blots were probed with anti-HA and anti-RPT5 antibodies. d Immunoblots showing native PIF1 level in spaQ mutant compared to wild type. e Immunoblots show that TAP-SPA1 can interact with PIF1-HA in a red light-inducible manner in vivo