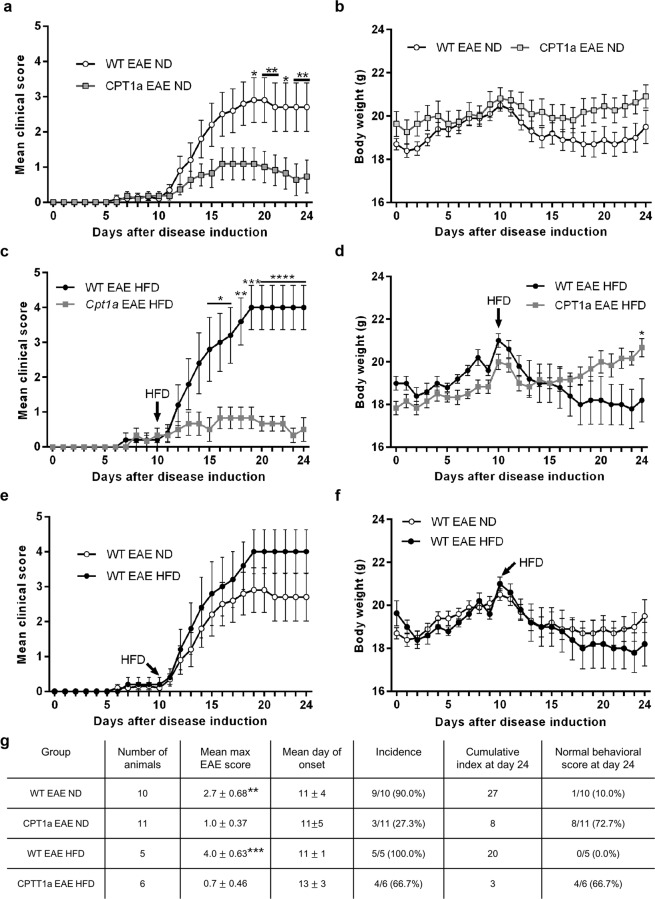
Figure 1.
The effect of a CPT1 mutation in an EAE model in normal (ND) and high fat (HFD) diet. WT and Cpt1a P479L mice were immunized with MOG35-55 and received either ND throughout the experimental period or were introduced to HFD on the 10 day of the study. The mean clinical score was overall higher in WT EAE ND (n = 10) mice than in Cpt1a P479L EAE ND (n = 11) mice, and from day 19 and further, the difference between both groups was significant (a). No significant differences in the average body weight were observed between WT EAE ND mice and Cpt1a P479L EAE ND mice. However, the WT EAE ND animals were characterized with a tendency of generally lower body weight especially in the last week of the experiment (b). WT EAE HFD mice (n = 5) show significantly higher clinical scores compare to Cpt1a P479L EAE HFD mice (n = 6) from the 15 day of the experiment and onward (c). The weight of WT EAE HFD animals started a constant decline after introduction of HFD on day 10 of the experiment and reached significantly different level compare to Cpt1a P479L EAE HFD on the 24 day of the study (d). The comparison of WT EAE ND and WT EAE HFD animals did not reveal any significant differences. However, a clear tendency of worst clinical scores and lower body weight of HFD receiving animals was noted (e,f). Classical EAE parameter of the mean maximum score was significantly lower in Cpt1a P479L EAE ND mice versus WT EAE ND mice (g). Similarly the WT EAE HFD group was characterized with much higher mean max EAE score of 4, whereas, CPT1a EAE HFD animals appeared to be in much better condition with mean EAE score of 0.7 (g). Data in panels a–f analyzed with RM two-way ANOVA with Sidak’s multiple comparisons post hoc test, data in panel g analyzed with unpaired t tests. Data are presented as the mean ± SEM. Asterisks indicate the level of statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).