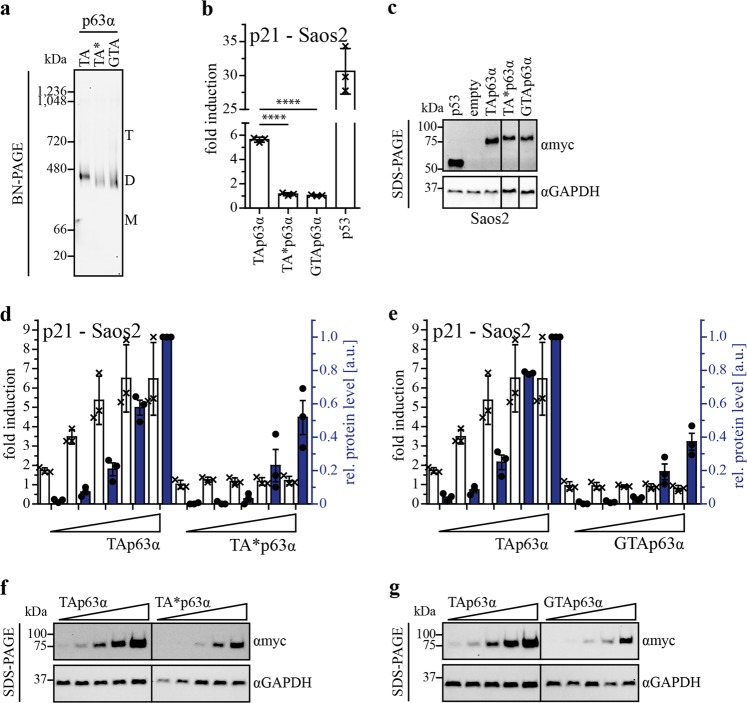
Fig. 2. Oligomeric conformation and transactivation potential of TA*p63α and GTAp63α.
a BN-PAGE analysis of myc-tagged TAp63α, TA*p63α, and GTAp63α. Three hundred nanogram expression vector carrying the p63 gene were transiently transfected in H1299 cells (12-well plate). Cells were harvested 24 h after transfection. Migration of the different oligomeric states is indicated by T (tetramer), D (dimer), and M (monomer). For all three isoforms only dimers are detectable. Western blots were performed with an anti-myc antibody (4A6, Merck). b Transactivation (TA) assay of TAp63α, TA*p63α and GTAp63α wt, on the p21 promotor. p53 was used as positive control. Hundred nanogram of each plasmid (pcDNA3, pGL3 and pRL-CMV) were transiently transfected in Saos-2 cells (12-well plate). Cells were harvested 24 h after transfection and assay was performed. Bars represent the mean value of the biological triplicate, error bars represent the standard deviation (SD), crosses represent the mean value of the technical replicates (c) SDS-PAGE followed by immunoblotting for myc-tagged TAp63α, TA*p63α, GTAp63α, and p53 protein levels of the TA assay performed on p21 promotor in Saos-2 cells (Fig. 2b). GAPDH was used as loading control. d, e TA titration assay of TA*p63α and GTAp63α in comparison to TAp63α on the p21 promotor. Hundred nanogram of pGL3 and pRL-CMV and increasing amount of p63 DNA (10 ng, 25 ng, 50 ng, 100 ng, 150 ng) were transiently transfected in Saos-2 cells. Empty vector was added to a total amount of 350 ng DNA for transfection (12-well plate). Cells were harvested 24 h after transfection and assay was performed. Protein levels were determined by western blotting. Fold induction is indicated with white bars, relative protein level with blue colored bars. The protein level for the highest DNA amount of TAp63α was set to 1. Dots represent protein level of each biological triplicate. f, g SDS-PAGE followed by western blotting for myc-tagged TAp63α, TA*p63α and GTAp63α protein level in the TA titration assay performed on the p21 promotor in Saos-2 cells (Fig. 2c, d). GAPDH was used as loading control