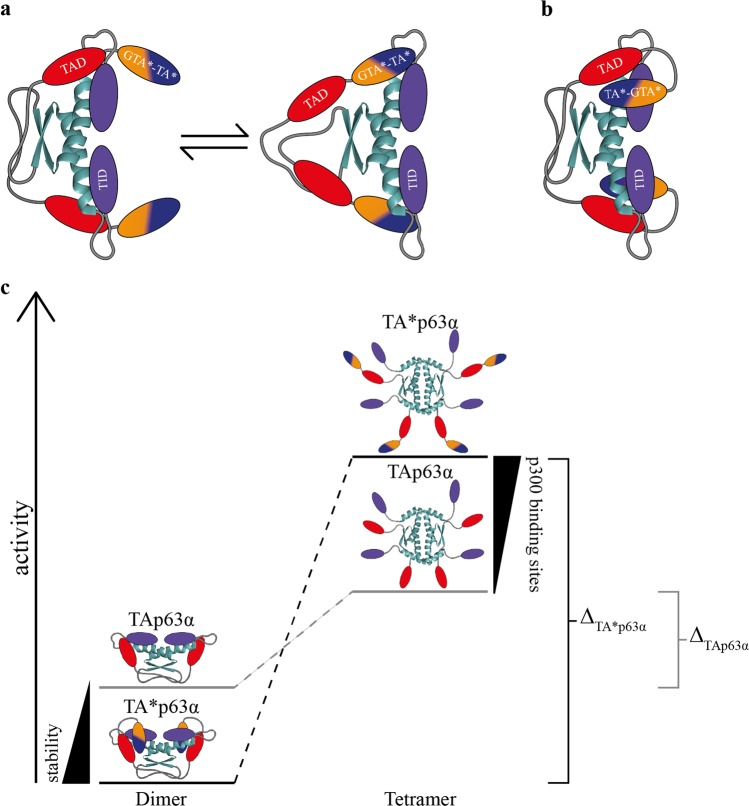
Fig. 7. Model of the regulatory function of the N-terminal specific peptides in TA*p63α. Models are shown explicitly for TA*p63α but represent GTAp63α as well.
a In the first model, TA*p63α’s and GTAp63α’s specific N-termini bind to the same interaction site as the TAD (red) on the TD (cyan). Consequently, the dimeric conformation has an additional “safe lock”. In case of dissociation of the TAD, the TA*-GTA* peptide (blue-orange) is able to keep the protein in its dimeric state and the TAD in close proximity for rebinding. b In the second model, TA*p63α’s and GTAp63α’s specific peptides are binding in their inactive, dimeric state to a further interaction site independent of the TAD keeping p63 in a more tightly packed state by building a “double lock” safety mechanism. c Schematic representation of the activity difference between the two oligomeric states of TA*p63α (GTAp63α) and TAp63α. The dimeric conformation of TA*p63α shows a higher stability due to the additional stabilization of the N-terminal peptides in comparison to TAp63α. The open, tetrameric state of TA*p63α, however, possess at least four further binding sites for the co-activator p300, resulting in a greater transactivation potential. Consequently, the activity difference between the inactive and active state of TA*p63α (ΔTA*p63α, black) is higher than the one of TAp63α (ΔTAp63α, gray)