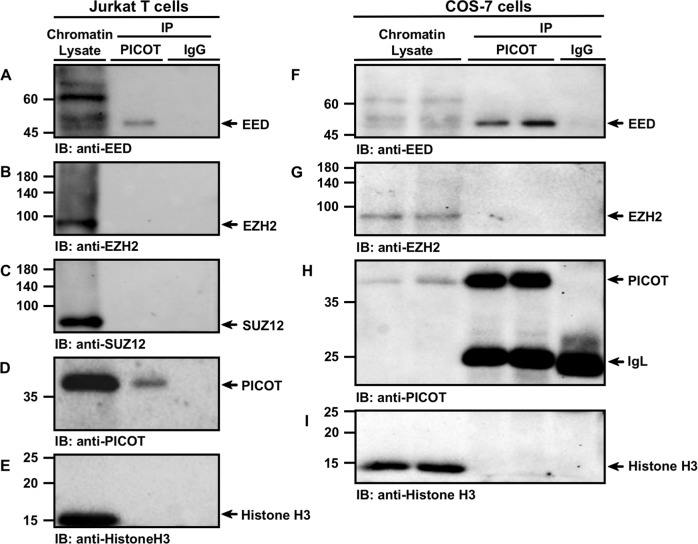
Fig. 3. PICOT-EED interaction co-exists in the chromatin compartment.
Jurkat and COS-7 cells (20 × 106/group) were fixed in 1% formaldehyde for 10 min at room temperature and chromatin lysates were prepared using the protein-protein ChIP protocol. The chromatin samples were precleared on protein G-sepharose beads, incubated overnight at 4 °C with protein G sepharose bead-immobilized mouse anti-PICOT mAbs or mouse normal IgG (10 × 106 cell equivalent per group). Proteins were then eluted from the beads by resuspension of the samples in β-mercaptoethanol-containing sample buffer (180 µl) and boiling for 30 min. Following centrifugation, supernatants (1.5 × 106 cell equivalent in 20 µl) were subjected to SDS-PAGE on 10% gel under reducing conditions. Chromatin lysates (5 µg/lane) were boiled and electrophoresed in parallel. Proteins were then electroblotted onto nitrocellulose membranes that were sequentially immunoblotted with rabbit anti-EED polyclonal Abs (a, f) and rabbit anti-EZH2 mAbs (b, g). A parallel membrane was sequentially immunoblotted with rabbit anti-SUZ12 mAbs (c), mouse anti-PICOT mAbs (d, h) and mouse anti-histone H3 mAbs (e, i). Protein bands were then visualized using the immunoperoxidase ECL detection system and autoradiography. Immunoprecipitation using IgG mAbs served as a negative control. The position of specific protein bands is indicated by arrows. Results are representative of three independent experiments. IP, immunoprecipitation; IB, immunoblot