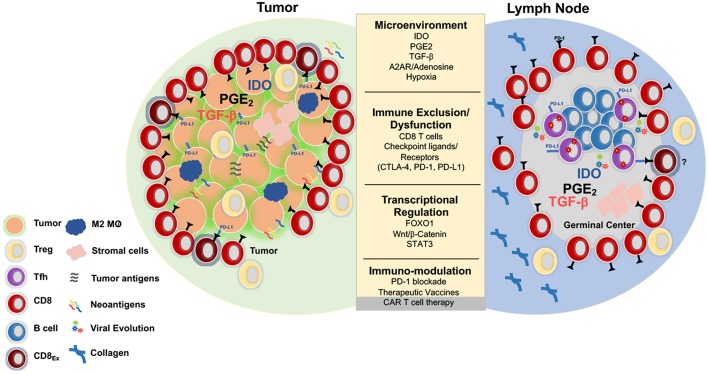
Figure 1.
Parallels between immunoregulation in solid tumors and lymph nodes. Tumors and LNs are composed of stromal and immune cells that secrete cytokines and growth factors such as transforming growth factor b (TGFβ), prostaglandin E2 (PGE2), indolamine 2-3-dioxygenase (IDO), and adenosine that shape the tumor and LN microenvironment and collectively contribute to suppression of the T cell response. Adenosine signals through the adenosine 2A receptor (A2AR) and promotes production of cyclic AMP, which impairs T cell trafficking, proliferation, and cytotoxicity (73). Immunosuppression is also induced by regulatory T cells (Treg) that express higher levels of CTLA-4, an inhibitory receptor that outcompetes CD80/CD86 on the surface of effector cells and promotes the production of IDO, an enzyme that degrades tryptophan and leads to impaired proliferation and Treg differentiation. Additionally, Tregs express high levels of CD73/CD39, enzymes that convert ATP to adenosine, which inhibit immune function (74, 75). Tumors upregulate inhibitory ligands such as PD-L1 that bind to inhibitory receptors resulting in suppression of adaptive immune responses (75). Several strategies have been developed in the immune-oncology field to overcome these barriers such as checkpoint blockade, small molecule inhibitors, therapeutic vaccines, and CAR T cell therapy. These immune based therapies can all be extended to the HIV cure field. Several mechanisms that result in resistance to checkpoint blockade and CD8 T cell exclusion include activation of the WNT/β -catenin pathway (76), localization of M2 macrophages within the tumor (77), and the secretion of TGFβ (78).